Difference between revisions of "Retrieve Protocol for Execution"
Dwhittenburg (talk | contribs) |
Dwhittenburg (talk | contribs) |
||
| Line 25: | Line 25: | ||
==Meeting Notes== | ==Meeting Notes== | ||
[[RPEMeetingNotesArchive|RPE Meeting Notes Archive]] | [[RPEMeetingNotesArchive|RPE Meeting Notes Archive]] | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
'''01/30/09''' | '''01/30/09''' | ||
Revision as of 15:55, 5 February 2009
Introduction
This is a draft of the Retrieve Protocol for Execution Profile (RPE) supplement to the Quality, Research, and Public Health (QRPH) Technical Framework. This draft is a work in progress, not the official supplement or profile.
Profile Abstract
The Retrieve Protocol for Execution Profile (RPE) provides an automated mechanism for EHR to retreive a complex set of clinical research instructions (or a protocol) from an EDC system to execute within the EHR.
- RFD scenario teams:
- Sponsors: Pfizer, Lilly, Novartis, Genzyme
- EHRs: Greenway, Cerner, Allscripts, Epic
- eClinical: Outcome Sciences, Nextrials, Phoenix Data Systems
- Proposal Editor: Landen Bain (CDISC)
- Profile Editors:
- Daemon Whittenburg (Greenway Medical Technologies)
- Diane Wold (Glaxo Smith Klein - Chair of CDISC’s Trial Design Team )
- Dan Levy (Outcome Sciences)
- Robert Barr (NexTrials)
- Chris Connor (Phoenix Data Systems / Bio-Imaging Technologies)
- Jane Griffin (Cerner)
- Jaime Lucove (AllScripts)
- Lisa Chatterjee (DIFZ, chair of CDISC’s Protocol Representation team)
- David Handelsman (SAS)
Meeting Notes
01/30/09
- Agenda
- Review input/output data elements needed for the UpdateProtocolManager(EnrollPatientRequest) transaction
- Work will be needed form the eClinical side in order to define these data elements
- What data is needed from the EHR to successfully Enroll a subject into EDC for a study
- What data is available from the EDC to store within the EHR
- [To be done after the input/output data elements are defined] Discuss XML standards to be used for message parameter supplied in the UpdateProtocolManager transaction
- Choosing an XML standard cannot happen until we have defined the data elements needed for the transaction
- Need to also think about other data elements needed for the other options for this "UpdateProtocolManager" transaction
- Introduce BRIDG (Biomedical Research Integrated Domain Group) as a standard to use with RPE
- Think of it in the same way as a content profile (CRD) for the transmission mechanism (RFD)
- caBIG
- Go over Prerequisites/Dependencies
- How to handle this section? Dependency for the RPE profile or Dependency for each transaction?
- I think we need a dependency for each transaction
- Open/Closed issues needs work?
- Discuss additional transaction or use of current ones that may need to be needed in the flow diagram
- Add UpdateProtocolState(SubjectComplete) transaction
- Landen updated the group on information about CRD CP discussion and RFD changes
- Make RFD and RPE abstract patterns for workflow management
- Use BPEL
- Landen to follow up with leads
- Possibly set up call with someone to discuss with the group
- Removed a few meeting dates for the upcoming month. We will meet on 2/6, 2/20 and 3/6
02/06/09
- Discussed confusion of RetrieveProtocolDefList as a transaction
- Is it a retrieve mechanism or a query mechanism?
- It is a query mechanism
- Need to have a defined starting point for the RPE profile
- That starting point will be the RetreiveProtocolDef transaction
- Discuss Proposal for the issue involving the RetrieveProtocolDefList transaction
- Combine the RetrieveProtocolDefList transaction into the RetrieveProtocolDef transaction
- The parameter passed for the RetrieveProtocolDef would be an XML structure for querying the ProtocolManager
- For the use of retrieving a protocolDef from the ProtocolManager the query would be for a specific protocolDef
- In the future this could be expanded to provide the ability to query on multiple parameters
- Those mutliple parameters being anything included in the TDM
- Including a way to request a list of protocolDefs
- For now the protocolDefID needed for the RetreiveProtocolDef transaction would be supplied similar to how the formIDs are provided via RFD
- This allows us to focus on the transactions from UpdateProtocolExeStep(EnterPatientRequest) to UpdateProtocolExeStep(EnrollPatientRequest)
- Is it a retrieve mechanism or a query mechanism?
- Removed EDC and EHR from RPE Flow Diagram
- Split scheduling UpdateProtocolExeStep transaction into two different transactions
- UpdateProtocolExeStep(PatientScheduled) - Patient visits are scheduled
- UpdateProtocolExeStep(RecordPatientVisit) - Patient has came in for visit[n]
- Need a transaction for 'Study has been shutdown, what needs to take place as far as patient's state and protocolDef within EHR?'
- Items discussed at the 01/27/2009 IHE face-to-face meeting
- Define the possibilities of getting back a NACK from UpdateProtocolExeStep...(screen failed)
- Add prerequisite to UpdateProtocolExeStep for Enter (consent)
- Batch enter patientIDs into study?
- define transactions between enter and enroll (screening?) (RFD?)
- UPES(PatientIdentified) take out?
- screen failed message both ways?
- candidateID for EnterPatientRequest?
- ability for EHR to submit the SubjectID (Psuedonemized)?
- change name of UpdateProtocolExeStep to ExecuteProtocolStep?
- Research BPEL as the standard to use for the Workflow Management transactions
- re-use workflow standards
- resource - Karen (IBM)
- Does the profile need to be more generic so that it can be used for other workflows than just clinical research?
- ProtocolManager to WorkflowManager?
- UpdateProtocolExeStep to UpdateWorkflowStep/ExecuteWorkflowStep?
- Is every Friday at 1pm (est) a good time to meet or should we stretch it out to every 2 weeks for a couple of months...lots of work to do...
- Add additional use cases, such as the Cancer Registry's use case
Open Issues
Open issues are being tracked in the meeting notes in red
- Similar issues are addressed in the Performance Measurement Data Element Structured for EHR Extraction white paper. What relation does RPE have with quality initiatives?
- Move "pre-order labs and other assessments" to "Patient Involvement" in use case graphic
Closed Issues
Closed issues are being tracked in meeting notes in green
- How to deal with protocols amendments taken during the study?
- Provided the AmendProtocolDef transaction
- How does RPE relate to RFD. Is RPE essentially a content profile using RFD infrastructure? Or does RFD create new RFD-like transactions.
- RPE is a set of transactions that allow communication between a ProtocolManager and a ProtocolExecutor relating to the ProtocolDef. RFD is a communication mechanism that will be referenced and used within RPE
- How much automation of protocol events is within grasp? How to executable tasks get conveyed to the Protocol Executor (Enabler? Enactor?)
- We have taken a look at as much automation as possible with the RPE profile. We will focus on the UpdateProtocolExeStep transaction, specifically for the EnrollPatientRequest message
- The executable tasks get conveyed to the ProtocolExecutor via the CDISC Trial Design Model standard
- Find a correct place for (Actions before Protocol Executor agrees to participate - approval from IRB, Form5272 for investigator, training, contract, site signs NDA with pharma company.
- These should become dependencies for the AgreeToParticipateProtocolDef transaction
- Change "Enroll in Protocol" transaction to be "Agrees to Participate".
- changed in profile and RPE Flow Diagram
- Remove "Review CRFs for data capture and data entry" from Use Case Graphic
- Removed, but not using that Graphic currently for workflow
- Add transactions for
- Study changes, schedule is updated, version each study, check study version transaction
- AmendProtocolDef
- Protocol Manager Submit Alert to Protocol Executor
- AlertProtocolState
- Schedule visits transaction (needs to be a seperate transaction after EnrollPatient)
- UpdateProtocolExeStep(PatientScheduled)
- Study changes, schedule is updated, version each study, check study version transaction
Risks
- Cross-system workflow integration is a relatively new area for IHE.
- The EHRs risk encountering the clinical research regulatory environment 21 CFR part 11.
Summary
Many healthcare sites supplement their core patient care activities by participating in clinical trials. Currently, the tasks required for clinical research participation are served by systems entirely separate from the site's EHR. The ITI profile Retrieve Form for Data-capture (RFD), along with its complementary content profile Clinical Research Data-capture, have set a path towards integrating site-based clinical research workflow into the task manager of an electronic health record. This new profile, Retrieve Protocol for Execution, expands the scope of workflow integration between clinical research and EHR systems.
CDISC's Protocol Representation team intends to develop a standard protocol document, derived from the BRIDG, a RIM-linked data model. This protocol representation includes the Trial Design Model a standard structure for representing the planned sequence of events and the treatment plan of a trial. This planned sequence of events includes many tasks that could be executed by an EHR's workflow engine. The 'schedule of activities' section of the trial design includes clinical trial activities such as interventions (e.g., administering drug, surgery) and study administrative activities (e.g,. obtaining informed consent, distributing clinical trial material & diaries, randomization) as well as assessments. The time is ripe to insert these executable workflow tasks into the EHR for execution within the site's normal way of doing business.
The Problem
Research protocols are complex instruction sets that guide the conduct of trials. A subset of the protocol pertains to the activities of the healthcare provider site that participates in the trial. This instruction set specifies the data to be captured, tests to be ordered, inclusion and exclusion criteria for subjects, number and type of visits, etc. These instructions currently reside in hard copy binders which provide guidance for study coordinators at research sites. What is desired is a way to insert protocol instructions into an EHR for automatic completion.
Glossary
- ProtocolDef
- The protocol documentation created by eClinical that defines a clinical research study. The ProtocolDef will be maintained by the ProtocolManager.
- ProtocolState
- The protocol state at the point in which a patient is involved in a clinical study.
Volume I
Dependencies
- patient inclusion/exclusion criteria
- patient signed inform consent
- labs
- documented signed inform consent
- screening
Overview
Scope
Example
Use Case - Investigational New Drug Clinical Trial
In the uses cases below, we describe the before and after effects of implementing the Retrieve Protocol for Execution profile.
- The setting for the clinical trial use case is a physicians’ practice where patient care is delivered side-by-side with clinical research.
- The site, Holbin Medical Group, is a multi-site physician practice, employing over 100 physicians in a variety of specialties.
- Holbin’s CEO encourages the physicians to participate as site investigators for pharmaceutical-sponsored clinical trials.
Before Retrieve Protocol for Execution
- Preconditions
- A Clinical Research Protocol is defined by a clinical trials expert.
- Holbin provides support for clinical research activities in the form of a Research Department of twelve dedicated study coordinators, mostly RNs, along with clerical and data-entry support personnel.
- Holbin Medical Group uses an Electronic Health Record (EHR) and a number of sponsor-provided Electronic Data Capture (EDC) systems for documenting clinical trial activities.
- Use Case
- Clinical Research Site's Involvement
- Holbin’s involvement in a clinical study begins when the Research Department receives a request for proposal (RFP) from PharmaGen, a biopharma research sponsor.
- A Study Coordinator, Patricia Zone, RN, evaluates the RFP for business viability and clinical appropriateness, provides the requested documentation back to the sponsor, and agrees to participate
- Approved as a site for PharmaGen #1234 trial
- After being approved as a site for the PharmaGen #1234 trial, the site Holbin Medical Gruop provides the required regulatory documentation to the sponsor
- The physician identified as the Principal Investigator and other study personnel receive protocol-specific training from the sponsor, including training in use of the SynerGen EDC system.
- During the trial set-up period, Patricia takes a number of steps that require interaction with the EHR. At this juncture, searches are at an aggregate level
- Ensures that the appropriate system security is in place for this protocol;
- Recruits patients to participate as subjects according to inclusion and exclusion criteria described in the study protocol;
- Creates a visit type for 1234 patient visits;
- Reviews CRFs for data capture and data entry;
- Pre-orders labs and other assessments;
- Performs all the attendant financial tasks.
- Following set up, Patricia contacts Corey Jones, a patient at Holbin, about participating in the trial, and Corey agrees to participate as a subject. A number of tasks deal with this individual patient
- Register Corey in the EHR as a subject in trial #1234, using the EHR’s patient index.
- She also registers Corey as a subject in the EDC system.
- She schedules Corey’s study visits using the EHR scheduling module, and flags the visits as pertaining to the trial #1234.
- Initiates clinical trial care and trial-specific documentation.
- Postconditions
- Holbin Medical Group uses an Electronic Health Record (EHR) and the SynerGen EDC Electronic Data Capture (EDC) system to document the PharmaGen #1234 trial activities.
After Retrieve Protocol for Execution
- Precondition
- A Clinical Research Protocol is defined by a clinical trials expert.
- Use Case
- Clinical Research Site's Involvement
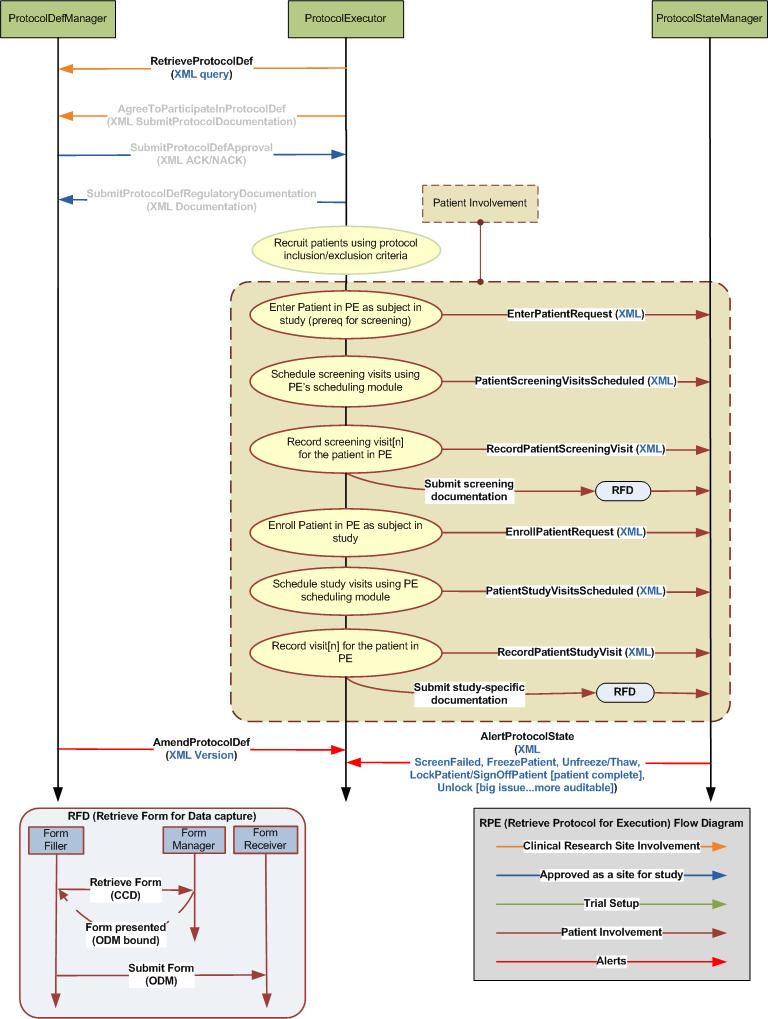
- ProtocolExecutor uses the RetrieveProtocolList transaction to obtain a list of protocols from the Protocol Manager
- ProtocolExecutor uses the AgreesToParticipate transaction to notify ProtocolManager that the site agrees to participate in the study
- ProtocolExecutor uses the RetrieveProtocol transaction to obtain the specific protocol from the ProtocolManager
- Approved as a site for PharmaGen #1234 trial
- ProtocolManager uses the SubmitProtocolApproval transaction to the ProtocolExecutor for a specific protocol
- ProtocolExecutor uses the SubmitRegulatoryDocumentation transaction to submit required regulatory documentation to the ProtocolManager
- Trial Setup
- ProtocolExecutor uses the UpdateProtocolManager (patient identified) transaction to let the ProtocolManager know that patients have been identified
- Patient Involvement
- ProtocolExecutro uses the UpdateProtocolManager (request enroll patient) transaction to attempt to enroll the patient into the study
- ProtocolExecutor uses the UpdateProtocolManager (schedule patient study visits) transaction to update the ProtocolManager that the study visits have been scheduled
- Postcondition
- Holbin Medical Group uses an Electronic Health Record (EHR) to document the PharmaGen #1234 trial activities using RFD.
Actors/Transaction
Process/Flow
Actors
- Protocol Executor
- An entity wanting to access clinical research protocols from a seperate entity that manages clinical research protocols.
- An example would be an EHR vendor that wants to participate in clinical studies by accessing clinical research protocols.
- Protocol Manager
- An entity that manages clinical research protocols.
- An example would be an EDC vendor that wishes to allow access to the list of clinical research protocols contained within the EDC system.
Transactions
- RetrieveProtocolDefList
- Used to allow the ProtocolExecutor to retrieve a list of ProtocolDefs from the ProtocolManager.
- Can be used by the site trying to view potential studies that the site may want to be involved in.
- Need to discuss another transaction that would allow the ProtocolManager to publish a ProtocolDef to the ProtocolExecutor as it is added (ProtocolManager PublishProtocolDef to ProtocolExecutor???). An alert when a new ProtocolDef is available.
- AgreeToParticipateInProtocolDef
- Used to allow a ProtocolExecutor to notify the ProtocolManager the intent to participate in a particular study
- Can be used by the site attempting to enroll in a clinical study
- RetreiveProtocolDef
- Used to allow a ProtocolExecutor to retrieve one instance of a ProtocolDef for a particular study
- Can be used by a site attempting to pull down a ProtocolDef once they have finalized all Prerequisites/Dependencies to execute this transaction
- SubmitProtocolDefApproval
- Used to allow a ProtocolManager send an approval that the ProtocolExecutor has been approved for a ProtocolDef
- Can be used by an eClinical vendor to alert a clinical site that they have been approved for a particular study
- SubmitProtocolDefRegulatoryDocumentation
- Used to allow a ProtocolExecutor to send Regulatory Documentation to a ProtocolManager
- Can be used by a clinical site to submit regulatory documentation for a specific protocol after being approved for participation to the eClinical vendor
- UpdateProtocolExeStep
- Used to allow a ProtocolExecutor to update a ProtocolManager when an execution step has been taken
- Can be used by a clinical site to update an eClinical vendor when an execution step has been taken for a particular ProtocolDef
- Message Options
- PatientIdentified
- EnterPatientRequest/ScreenPatientRequest
- EnrollPatientRequest
- PatientScheduled
| Actor | Transaction | Option | Section |
| Protocol Executor | RetrieveProtocolDefList | ? | ? |
| AgreeToParticipateInProtocolDef | ? | ? | |
| RetrieveProtocolDef | ? | ? | |
| SubmitProtocolDefApproval | ? | ? | |
| SubmitProtocolDef | ? | ? | |
| UpdateProtocolExeStep | R | ? | |
| Protocol Manager | RetrieveProtocolDefList | ? | ? |
| AgreeToParticipateInProtocolDef | ? | ? | |
| RetrieveProtocolDef | ? | ? | |
| SubmitProtocolDefApproval | ? | ? | |
| SubmitProtocolDef | ? | ? | |
| UpdateProtocolExeStep | R | ? |
Volume II
Retrieve Protocol for Execution Content
Standards
Interaction Diagrams
Resources
Return to Quality, Research and Public Health Domain Main Page
Return to Quality, Research and Public Health Planning Committee
Return to Quality, Research and Public Health Technical Committee