PCC TF-1/CPR
Volume 1
HIMSS and RSNA
Integrating the Healthcare Enterprise
IHE Patient Care Coordination
Cancer Registry Pathology Report (CPR)
Technical Framework Supplement
Volume I
Revision 3.0
2008-2009
Public Comment
- (PCC TF-1/Preface)Preface to Volume I of the PCC Technical Framework
- (PCC TF-1/Introduction)Introduction to the PCC Technical Framework
- (PCC TF-1/About)About the Patient Care Coordination Integration Profiles
- (PCC TF-1/Dependencies)Dependencies of the PCC Integration Profiles
- (PCC TF-1/Overview)PCC Integration Profiles Overview
- (PCC TF-1/History)History of Annual Changes
- (PCC TF-1/Product Implementations)Product Implementations
Introduction
This is a draft of the Cancer Registry Pathology Report Content Profile (CRPR) supplement to the PCC Technical Framework. This draft is a work in progress, not the official supplement or profile.
Profile Abstract
The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Glossary
- International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3)
Classification system for reporting incidences of malignant diseases.
- NAACCR
The North American Association of Central Cancer Registries. A collaborative umbrella organization for cancer registries, governmental agencies, professional organizations, and private groups in North America interested in enhancing the quality and use of cancer registry data.
- Pathology Report
The written description of the microscopic examination of a tissue. The gross description reports the physical characteristics of the tissue: size, color, and abnormalities visible with the unaided eye. The microscopic description reports the cellular characteristics aided by the use of a microscope: what cells are involved, the behavior, and the aggressiveness or grade of any abnormality. The final diagnosis is a summary of the findings and indicates the pathologist’s impression of what was found in concise terms.
Issue Log
Open Issues
- Issue: Dependencies: need help understanding this component
- Depends on Care Management with the V2 option (and by reference ATNA and CT)
- Issue: Modify Actor/Transactions (Keith)
- Issue: Modify Content Module for V2 profiles (Keith)
- Issue: Grouping: Does this need to be modified for the CRC profile?
- Yes, not sure how
![]() -->>Register Pathology Implementation Guide with HL7 Message Profile Registry<<--
-->>Register Pathology Implementation Guide with HL7 Message Profile Registry<<--
- In Progress
Closed Issues
- Issue: Glossary: Use MERP glossary document or consolidated glossary tool?
- Refer to the existing document
- Issue: Options: need help understanding this component
- Won't have any
- Added options based on discussion at May f2f meeting.
- Issue: Transaction Definitions: need help understanding this component
- Not relevant to this profile
- Issue: What information do we need to include in 3.1.2, 3.1.3, 3.2, 3.3, 3.4?
- Just reference the NAACCR Documentation on this section
- Should we include a sample message?
- include a link to a webpage of a sample message. There are ways to convert a document to a webpage....
Volume I
Add the following bullet to the list of profiles
- The Cancer Registry Pathology Report Content Profile (CPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Dependencies
Add the following row(s) to the list of dependencies
| Integration Profile | Dependency | Dependency Type | Purpose |
|---|---|---|---|
| Cancer Registry Pathology Report (CPR) | Care Management (CM) | The Content Creator actor of the CPR profile must be grouped with the Clinical Data Source Actor of the CM profile | The CPR profile defines the content sent in the PCC-11 transaction specified in the CM profile |
| Cancer Registry Pathology Report (CPR) | Care Management (CM) | The Content Consumer actor of the CPR profile must be grouped with the Care Manager Actor of the CM profile | The CPR profile defines the content recieved in the PCC-11 transaction specified in the CM profile |
| Cancer Registry Pathology Report (CPR) | ATNA | Actors the CPR profile shall implement the Secure Node Actor of the ATNA profile | Ensures that transmissions and changes to patient health information are logged in an audit repository, and that communication is secured between nodes. |
| Cancer Registry Pathology Report (CPR) | ATNA | Actors the CPR profile shall implement the Time Client Actor of the CT profile | Ensures that concistent time is used in all messages. |
Cancer Registry Pathology Report Content
The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Monitoring the occurence of cancer is a cornerstone of cancer control decision making. this monitoring, referred to as cancer surveillance, can be used to trigger case investigations, follow trends, evaluate the effectiveness of prevention measures such as screening and early detection programs and suggest public health priorities. It is vital to identify and registrar all cancer cases. By not identifying all of the cancer cases, cancer incidence will be underestimated, giving a false impression of the magnitude of the cancer problem in the registry’s population area. Inaccurate incidence rates can misdirect cancer control efforts, and provide a false picture of the effectiveness of treatment efforts.
Because most cancers are definitively diagnosed by microscopic examination of tissue, cancer surveillance programs rely on pathology reports to identify new cases and collect further information on cases previously reported.
Two challenges relate to pathology laboratory reporting of cancer cases. Laboratories may photocopy selected pathology reports and mail them to the cancer registry. This method is labor intensive and prone to missing cases because the laboratory staff is not aware of the full case definition for identifying a cancer case. Additionally, the cancer registry staff must re-enter the information into the cancer registry with a risk of data entry errors.
Alternately, laboratories may choose not to actively report cases, but allow cancer registry personnel to identify and photocopy appropriate pathology reports on site. This is very costly for the registry and has the same risk of data entry errors when the reports are manually entered into the registry database.
Further information on the benefits, challenges and the cancer registry's uses of electronic pathology reporting can be found in the:
- [4] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
Use Cases
- Pre-condition
A woman goes in for a routine mammogram and a suspicious area is noted. The woman is referred by her family physician to a surgeon who does a biopsy and sends the tissue sample to the pathology laboratory for review and diagnosis.
The pathology laboratory receives the specimen, logs it into the laboratory information system (LIS) and prepares the specimen for analysis. The pathologist analyzes the specimen and dictates his/her findings, which are then transcribed into the LIS. The pathologist verifies the accuracy of the report and signs the transcribed report. The LIS marks the pathology report as "Final", triggering the use case events.
- Events
- 1. Pathology Laboratory Creates Message
The LIS identifies that the report matches submission criteria because it is a histopathology test (biopsy) and the diagnosis relates to cancer ("carcinoma"). The LIS gathers information from its database and other appropriate databases to create a message that contains all of the required data elements. The LIS validates the data to ensure it conforms to the HL7 2.3.1 ORU-RO1 specification and transmits it to the Cancer Registry using a secure connection.
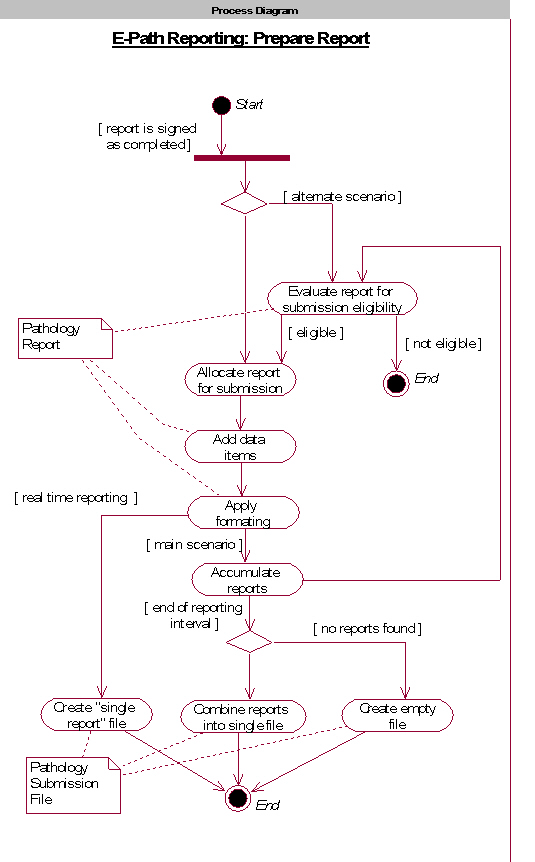
: [1] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
- 1. Cancer Registry Processes Registration
The Cancer Registry receives the HL7 messages, parses it into the processing database and determines that a cancer diagnosis has been documented. The Cancer Registry staff codes the medical information. The pathology data and coded information is exported to the Cancer Registry Database and the message is archived.

: [2] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
- Post-condition
Pathology information has been added to the Cancer Registry Database.
The message is available for future use as needed.
Scope
The following diagram constrains the scope of the Cancer Registry Pathology Report Content Profile.
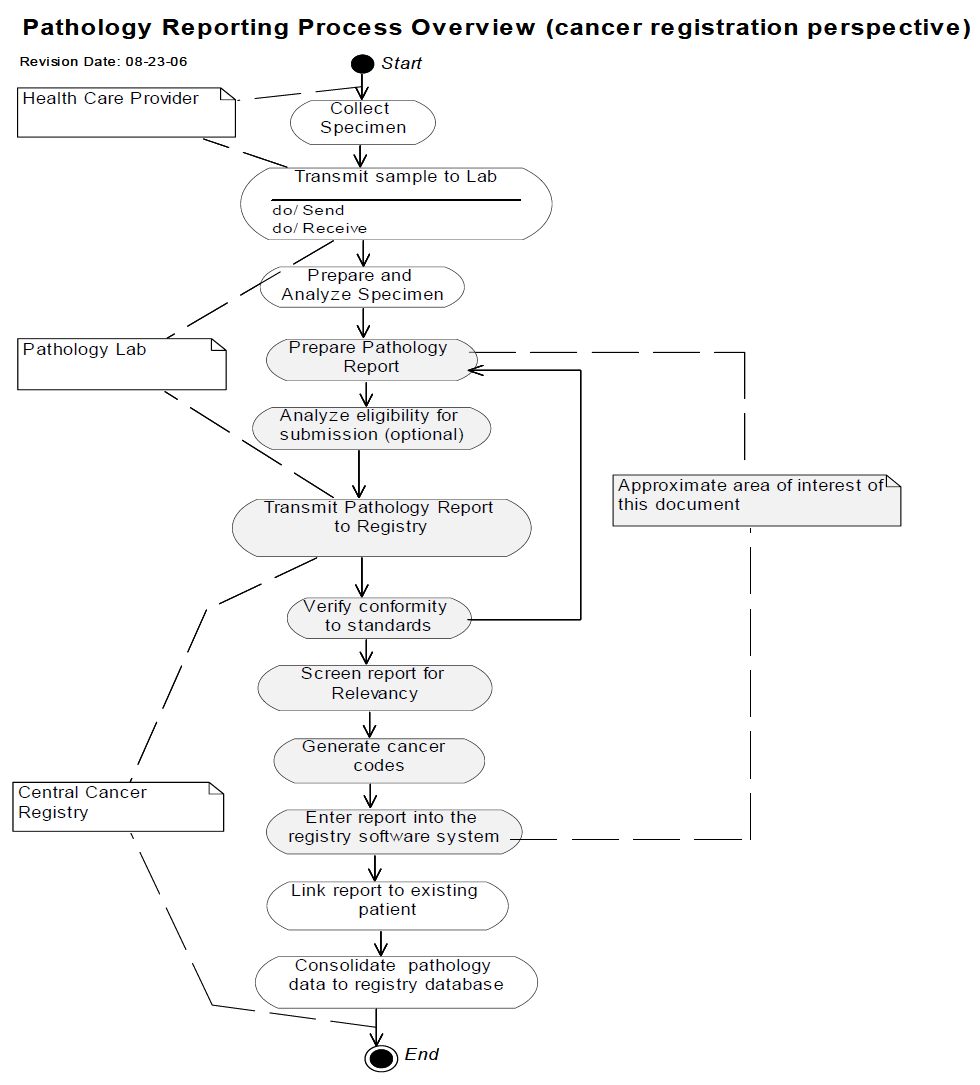
: [3] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
Actors/Transaction
There are two actors in this profile, the Content Creator and the Content Consumer. Content is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile.
Grouping
Care Management (CM)
The Content Creator of this profile must be grouped with the Clinical Data Source of the Care Management profile. The Clinical Data Source actor must implement the V2 Care Management Update Option.
The Content Consumer Actor of this profile must be grouped with the Care Manager actor of the Care Management profile.
Basic Patient Privacy Consents (BCCP)
When patient consent is required (for example, as in the case of Quebec), the Content Creator may be grouped with the Content Consumer Actor of the BPPC profile. When grouped with that actor, the Content Creator shall verify that an appropriate consent has been registered for the patient to share the pathology data prior to sending the message.
Policy Considerations
- Batch or Real Time Reporting
Because the Cancer Registry Pathology Report Content Profile relates to secondary use of data, Batch Reporting with a frequency of every 24 hours is recommended.
- Case Selection
A Content Consumer may require that all reports be transmitted, regardless of relevance to cancer, or it may require transmission to be restricted based on specific selection criteria. This is a policy decision made by the Content Receiver based on state, provincial and fedreal legislations and/or regulations, rules or by operating policy.
Security Considerations
Requirements/Capabilities
- Case selection
Provide option to select all reports or only those that meet selection criteria. Refer to Volume II for:
- List of anatomic pathology reports
- Standards for Identifying Pathology Reports
- List of Cancer Medicine Elisa kit
- Message Conformance
Message can be validated using:
- [5] : NAACCR Volume V Messaging Workbench Profile.
Appendix A - Actor Descriptions
Actors are information systems or components of information systems that produce, manage, or act on information associated with operational activities in the enterprise.
- Content Creator
- The Content Creator Actor is responsible for the creation of content and transmission to a Content Consumer.
- Content Consumer
- A Content Consumer Actor is responsible for viewing, import, or other processing of content created by a Content Creator Actor.
- Clinical Data Consumer
- A clinical data consumer makes use of clinical patient data.
- Clinical Data Source
- A Clinical Data Sources maintains patient information about vital signs, problem and allergies, results from diagnostic tests (e.g., Lab, Imaging, or other test results), medications, immunizations or historical or planned visits and procedures.
Appendix B - Transaction Descriptions
Transactions are interactions between actors that transfer the required information through standards-based messages. The PCC Technical Framework does not define any specific transactions, as these are assumed to be carried out through the use of transactions defined in other IHE Profiles.
- Query Existing Data
- Request information about recent patient information, used to obtain vital signs measurements, problems and allergies, diagnostic results, medications, immunizations, or procedures or visits relevant for a patient. The query may request information about some or all of the above topics, or may request information on a specific topic, or one entered for a specific encounter or date range.
Appendix C - How to Prepare an IHE Integration Statement
IHE Integration Statements are documents prepared and published by vendors to describe the conformance of their products with the IHE Technical Framework. They identify the specific IHE capabilities a given product supports in terms of IHE actors and integration profiles described in the technical frameworks of each domain.
Users familiar with these concepts can use Integration Statements to determine what level of integration a vendor asserts a product supports with complementary systems and what clinical and operational benefits such integration might provide. Integration Statements are intended to be used in conjunction with statements of conformance to specific standards (e.g., HL7, IETF, DICOM, W3C, etc.).
IHE provides a process for vendors to test their implementations of IHE actors and integration profiles. The IHE testing process, culminating in a multi-party interactive testing event called the Connectathon, provides vendors with valuable feedback and provides a baseline indication of the conformance of their implementations. The process is not intended to independently evaluate, or ensure, product compliance. In publishing the results of the Connectathon and facilitating access to vendors' IHE Integration Statements, IHE and its sponsoring organizations are in no way attesting to the accuracy or validity of any vendor's IHE Integration Statements or any other claims by vendors regarding their products.
IMPORTANT -- PLEASE NOTE: Vendors have sole responsibility for the accuracy and validity of their IHE Integration Statements. Vendors' Integration Statements are made available through IHE simply for consideration by parties seeking information about the integration capabilities of particular products. IHE and its sponsoring organizations have not evaluated or approved any IHE Integration Statement or any related product, and IHE and its sponsoring organizations shall have no liability or responsibility to any party for any claims or damages, whether direct, indirect, incidental or consequential, including but not limited to business interruption and loss of revenue, arising from any use of, or reliance upon, any IHE Integration Statement.
Structure and Content of an IHE Integration Statement
An IHE Integration Statement for a product shall include:
- The Vendor Name
- The Product Name (as used in the commercial context) to which the IHE Integration Statement applies.
- The Product Version to which the IHE Integration Statement applies.
- A publication date and optionally a revision designation for the IHE Integration Statement.
- The following statement: "This product implements all transactions required in the IHE Technical Framework to support the IHE Integration Profiles, Actors and Options listed below:"
- A list of IHE Integration Profiles supported by the product and, for each Integration Profile, a list of IHE Actors supported. For each integration profile/actor combination, one or more of the options defined in the IHE Technical Framework may also be stated. Profiles, Actors and Options shall use the names defined by the IHE Technical Framework Volume I. (Note: The vendor may also elect to indicate the version number of the Technical Framework referenced for each Integration Profile.)
Note that implementation of the integration profile implies implementation of all required transactions for an actor as well as selected options.
The statement shall also include references and/or internet links to the following information:
- Specific internet address (or universal resource locator [URL]) where the vendor's Integration Statements are posted
- URL where the vendor's standards conformance statements (e.g., HL7, DICOM, etc.) relevant to the IHE transactions implemented by the product are posted.
- URL of the IHE Initiative's web page for general IHE information www.himss.org/ihe.
An IHE Integration Statement is not intended to promote or advertise aspects of a product not directly related to its implementation of IHE capabilities.
Format of an IHE Integration Statement
Each Integration Statement shall follow the format shown below. Vendors may add a cover page and any necessary additional information in accordance with their product documentation policies.
| IHE Integration Statement | Date | 12 Oct 2005 |
| Vendor | Product Name | Version |
| Any Medical Systems Co. | IntegrateRecord | V2.3 |
| This product implements all transactions required in the IHE Technical Framework to support the IHE Integration Profiles, Actors and Options listed below: | ||
| Integration Profiles Implemented | Actors Implemented | Options Implemented |
| Cross-Enterprise Sharing of Medical Summaries | Document Consumer | View Option |
| Audit Trail and Node Authentication | Secure Node | none |
| Patient Identity Cross-referencing | Patient Identifier Cross-reference Consumer | PIX Update Notification |
| Internet address for vendor's IHE information:www.anymedicalsystemsco.com/ihe | ||
| Links to Standards Conformance Statements for the Implementation | ||
| HL7 | www.anymedicalsystemsco.com/hl7 | |
| Links to general information on IHE | ||
| In North America: www.ihe.het | In Europe: www.ihe-europe.org | In Japan: www.jira-net.or.jp/ihe-j |
IHE Integration Statement template
An IHE Integration Statement template (MS Word version) is available here.
The IHE Product Registry
The assumption of an integration statement is that all actors listed are functionally grouped and conform to any profile specifications for such groupings. In case of exceptions the vendor must explicitly describe the functional groupings.
IHE has developed a new Web-based database of Integration Statements. The IHE Product Registry enables developers to create, manage and publish Integration Statements for their commercial and open source healthcare IT systems. It allows users to browse for these systems based on their conformance with specific IHE Actors and Profiles. The system is open for use by developers and users now!
Appendix D - Braden Scale for Predicting Pressure Sore Risk
See File:Braden.pdf
Glossary
The following terms are used in various places within this technical framework, and are defined below. The complete IHE Glossary is available on the IHE Wiki at http://wiki.ihe.net/index.php/IHE_Glossary .
- Actor
- An entity within a use case diagram that can perform an action within a use case diagram. Possible actions are creation or consumption of a message
- Acuity Assessment
Also known as triage category, this is the acuity of the patient assigned during the process of ED triage. A number of evidenced based triage scales exist, including the Emergency Severity Index (ESI), Canadian Triage and Acuity Scale (CTAS), the Australasian Triage Scale (ATS), and the Manchester Triage System. In many emergency departments, patients may simply be classified as emergent, urgent or non-urgent.
- ADT
- Admit, Discharge & Transfer.
- Affinity Domain Policy
- Affinity Domain Policy that clearly defines the appropriate uses of the XDS Affinity Domain. Within this policy is a defined set of acceptable use Privacy Consent Policies that are published and understood.
- ASTM
- Formerly the American Society of Testing and Materials, now ASTM International. An SDO that develops a number of standards across a wide variety of industries, including healthcare.
- ATNA
- Audit Trail and Node Authentication. An IHE ITI profile.
- Care Context
- The participations surrounding the care provision act, and the attributes of that act. Everything in the document header. Data history, links to clinical reasoning.
- Continuity of Care Document(CCD)
- An HL7 Clinical Document Architecture (CDA) implementation alternative to ASTM ADJE2369 for institutions or organizations committed to HL7 standards. This specification was developed as a collaborative effort between ASTM and HL7. More information is available from http://www.hl7.org.
- Continuity of Care Record (CCR)
- A core data set of the most relevant administrative, demographic, and clinical information facts about a patient’s healthcare, covering one or more encounters. The CCR is Designation E2369-05 of the ASTM (American Society for Testing and Materials, International). More information is available from http://www.astm.org.
- Clinical Document Architecture (CDA)
- An HL7 standard for the exchange for clinical documents. It specifies the structure and semantics of clinical documents. More information is available from http://www.hl7.org.
- Content Binding
- A content binding describes how the payload used in an IHE transaction is related to and/or constrained by the data elements contained within the content sent or received in those transactions.
- CRS
- Care Record Summary. An implementation guide that constrains CDA Release 2 for Care Record Summary documents.
- CT
- Consistent Time Integration Profile.
- DICOM
- Digital Imaging and Communication in Medicine
- DSG
- Digital Signatures. An IHE ITI Profile.
- EDIS
- An Emergency Department Information System (EDIS) is an extended EHR system used to manage data in support of Emergency Department patient care and operations. The functions of an EDIS may be provided by a single application or multiple applications.
- eMPI
- Enterprise Master Patient Index.
- EMR
- Electronic Medical Record, an Electronic Health Record system used within an enterprise to deliver care (also called EHR-CR by IHE-XDS).
- Estimated Time of Arrival
- the time the patient being referred can be expected to arrive in the emergency department.
- EUA
- Enterprise User Authentication Integration Profile.
- Expected Actions
- Actions which should occur as the result of a trigger event.
- HIMSS
- Healthcare Information and Management Systems Society.
- HL7
- Health Level Seven
- HIS
- Hospital Information System.
- IHE
- Integrating the Healthcare Enterprise.
- Interaction Diagram
- A diagram that depicts data flow and sequencing of events.
- IT
- Information Technology.
- Logical Observation Identifiers Names and Codes (LOINC®)
- A vocabulary developed by the Regenstrief Institute aimed at standardizing laboratory and clinical codes for use in clinical care, outcomes management, and research. Additional information found at http://www.regenstrief.org/medinformatics/loinc/.
- Mode of Arrival
- The method of transportation used to transport the patient to the Emergency Department.
- MPI
- Master Patient Index.
- MRN
- Medical Record Number.
- NAV
- Notification of Document Availability
- OID
- Object Identifier. (See also 'Globally Unique Identifier').
- Patient Identifier Cross-reference Domain
- Consists of a set of Patient Identifier Domains known and managed by a Patient Identifier Cross-reference Manager Actor. The Patient Identifier Cross-reference Manager Actor is responsible for providing lists of "alias" identifiers from different Patient Identifier Domains.
- Patient Identifier Domain
- A single system or a set of interconnected systems that all share a common identification scheme for patients. Such a scheme includes: (1) a single identifier-issuing authority, (2) an assignment process of an identifier to a patient, (3) a permanent record of issued patient identifiers with associated traits, and (4) a maintenance process over time. The goal of Patient Identification is to reduce errors.
- Portable Document Format.
- PIX
- Patient Identifier Cross Referencing. An IHE ITI Profile.
- PDQ
- Patient Demographics Query. An IHE ITI Profile.
- PHR
- Personal Health Record
- Procedure
- In the context of a "Pre-procedure History and Physical," the "procedure" is a surgery or an invasive examination of a patient that is required by quality review organizations to be preceded by a pre-procedure assessment of procedure risk and anesthesia risk. This assessment is typically referred to as a "Pre-operative" or "Pre-procedure History and Physical."
- Process Flow Diagram
- A graphical illustration of the flow of processes and interactions among the actors involved in a particular example.
- Proposed disposition
- the intended disposition (i.e. admission to ICU, discharge to home, transfer to psychiatric hospital), if known, that the referring provider expects the patient will end up after the emergency department intervention.
- Referral Source
- An individual, group, or agency that determined the patient should seek care at the ED. Referral source may be used to determine appropriate discharge referrals and services, or to provide surveillance data for program and service planning, or to examine referral patterns.
- Role
- The actions of an actor in a use case.
- RSNA
- Radiological Society of North America.
- sig.
- A Latin abbreviation for signetur used to represent the instruction following the medication name.
- Scope
- A brief description of the transaction.
SNOMED-CT® A comprehensive clinical terminology, originally created by the College of American Pathologists (CAP) and, as of April 2007, owned, maintained, and distributed by the International Health Terminology Standards Development Organisation (IHTSDO), a non-for-profit association in Denmark. The CAP continues to support SNOMED CT operations under contract to the IHTSDO and provides SNOMED-related products and services as a licensee of the terminology. More information available from http://www.ihtsdo.org/ or the United States National Library of Medicine at http://www.nlm.nih.gov/research/umls/Snomed/snomed_main.html
- Transport Mode
- the method the patient employs, or is provided to get to the emergency department.
- Trigger Event
- An event such as the reception of a message or completion of a process, which causes another action to occur.
- UID
- Unique Identifier (See also Globally Unique Identifier).
- Universal ID
- Unique identifier over time within the UID type. Each UID must belong to one of specifically enumerated species. Universal ID must follow syntactic rules of its scheme.
- Use Case
- A graphical depiction of the actors and operation of a system.
- XUA
- Cross Enterprise User Authentication
- XDS
- Cross Enterprise Document Sharing
Volume 2
HIMSS and RSNA
Integrating the Healthcare Enterprise
IHE Patient Care Coordination
Cancer Registry Pathology Report (CPR)
Technical Framework Supplement
Volume II
Revision 3.0
2008-2009
Public Comment
- (PCC TF-2/Preface)Preface
- (PCC TF-2/Introduction)Introduction
Namespaces and Vocabularies
This section lists the namespaces and identifiers defined or referenced by the IHE PCC Technical Framework, and the vocabularies defined or referenced herein.
The following vocabularies are referenced in this document. An extensive list of registered vocabularies can be found at http://www.hl7.org/oid/.
| codeSystem | codeSystemName | Description |
| 1.3.6.1.4.1.19376.1.5.3.1 | IHE PCC Template Identifiers | This is the root OID for all IHE PCC Templates. A list of PCC templates can be found below in CDA Release 2.0 Content Modules. |
| 1.3.6.1.4.1.19376.1.5.3.2 | IHEActCode | See IHEActCode Vocabulary below |
| 1.3.6.1.4.1.19376.1.5.3.3 | IHE PCC RoleCode | See IHERoleCode Vocabulary below |
| 1.3.6.1.4.1.19376.1.5.3.4 | Namespace OID used for IHE Extensions to CDA Release 2.0 | |
| 2.16.840.1.113883.10.20.1 | CCD Root OID | Root OID used for by ASTM/HL7 Continuity of Care Document |
| 2.16.840.1.113883.5.112 | RouteOfAdministration | See the HL7 RouteOfAdministration Vocabulary |
| 2.16.840.1.113883.5.1063 | SeverityObservation | See the HL7 SeverityObservation Vocabulary |
| 2.16.840.1.113883.5.7 | ActPriority | See the HL7 ActPriority Vocabulary |
| 2.16.840.1.113883.6.1 | LOINC | Logical Observation Identifier Names and Codes |
| 2.16.840.1.113883.6.96 | SNOMED-CT | SNOMED Controlled Terminology |
| 2.16.840.1.113883.6.103 | ICD-9CM (diagnosis codes) | International Classification of Diseases, Clinical Modifiers, Version 9 |
| 2.16.840.1.113883.6.104 | ICD-9CM (procedure codes) | International Classification of Diseases, Clinical Modifiers, Version 9 |
| 2.16.840.1.113883.6.26 | MEDCIN | A classification system from MEDICOMP Systems. |
| 2.16.840.1.113883.6.88 | RxNorm | RxNorm |
| 2.16.840.1.113883.6.63 | FDDC | First DataBank Drug Codes |
| 2.16.840.1.113883.6.12 | C4 | Current Procedure Terminology 4 (CPT-4) codes. |
| 2.16.840.1.113883.6.257 | Minimum Data Set for Long Term Care | The root OID for Minimum Data Set Answer Lists |
| 1.2.840.10008.2.16.4 | DCM | DICOM Controlled Terminology; PS 3.16 Content Mapping Resource, Annex D |
| 2.16.840.1.113883.6.24 | MDC | ISO/IEEE 11073 Medical Device Nomenclature |
| 2.16.840.1.113883.3.26.1.5 | NDF-RT | National Drug File Reference Terminology (NCI version) |
| 2.16.840.1.113883.11.19465 | nuccProviderCodes | National Uniform Codes Council Healthcare Provider Terminology |
| 2.16.840.1.113883.6.255.1336 | X12DE1336 | Insurance Type Code (ASC X12 Data Element 1336) |
| 2.16.840.1.113883.6.256 | RadLex | RadLex (Radiological Society of North America) |
The IHE FormatCode vocabulary is now managed in an Implementation Guide published using FHIR.
- formal canonical URI https://profiles.ihe.net/fhir/ihe.formatcode.fhir/ValueSet-formatcode.html
- formal publication URL https://profiles.ihe.net/fhir/ihe.formatcode.fhir/
- FormatCode gitHub repository for source of the Implementation Guide can be used to register issues, or create pull requests for modifications. Formal governance is managed by ITI Technical Committee.
This FormatCode vocabulary represents:
- Code System 1.3.6.1.4.1.19376.1.2.3
- Value Set 1.3.6.1.4.1.19376.1.2.7.1
IHEActCode Vocabulary
| CCD | ASTM/HL7 Continuity of Care Document | |
| CCR | ASTM CCR Implementation Guide |
The IHEActCode vocabulary is a small vocabulary of clinical acts that are not presently supported by the HL7 ActCode vocabulary. The root namespace (OID) for this vocabulary is 1.3.6.1.4.1.19376.1.5.3.2. These vocabulary terms are based on the vocabulary and concepts used in the CCR and CCD standards listed above.
| Code | Description |
| COMMENT | This is the act of commenting on another act. |
| PINSTRUCT | This is the act of providing instructions to a patient regarding the use of medication. |
| FINSTRUCT | This is the act of providing instructions to the supplier regarding the fulfillment of the medication order. |
| IMMUNIZ | The act of immunization of a patient using a particular substance or class of substances identified using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| DRUG | The act of treating a patient with a particular substance or class of substances identified using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| INTOL | An observation that a patient is somehow intollerant of (e.g., allergic to) a particular substance or class of substances using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| SUBSTANCE | A qualifier that identifies the substance used to treat a patient in an immunization or drug treatment act. The substance is expected to be identified using a vocabulary such as RxNORM, SNOMED CT or other similar vocabulary and should be specific enough to identify the ingredients of the substance used. |
| SUBSTCLASS | A qualifier that identifies the class of substance used to treat a patient in an immunization or drug treatment act. The class of substances is expected to be identified using a vocabulary such as NDF-RT, SNOMED CT or other similar vocabulary, and should be broad enough to classify substances by mechanism of action (e.g., Beta Blocker), intended effect (Dieuretic, antibiotic) or ... |
| For Public Comment | What else needs to appear above for SUBSTCLASS? |
IHERoleCode Vocabulary
The IHERoleCode vocabulary is a small vocabulary of role codes that are not presently supported by the HL7 Role Code vocabulary. The root namespace (OID) for this vocabulary is 1.3.6.1.4.1.19376.1.5.3.3.
| Code | Description |
| EMPLOYER | The employer of a person. |
| SCHOOL | The school in which a person is enrolled. |
| AFFILIATED | An organization with which a person is affiliated (e.g., a volunteer organization). |
| PHARMACY | The pharmacy a person uses. |
PCC Content Modules
This chapter contains the various content modules and value sets that are used with IHE Patient Care Coordintation profiles and transactions.
Conventions
Various tables used in this section will further constrain the content. Within this volume, the follow conventions are used.
- R
- A "Required" data element is one that shall always be provided. If there is information available, the data element must be present. If there is no information available, or it cannot be transmitted, the data element must contain a value indicating the reason for omission of the data. (See PCC TF-2: 5.3.4.2 for a list of appropriate statements).
- R2
- A "Required if data present" data element is one that shall be provided when a value exists. If the information cannot be transmitted, the data element shall contain a value indicating the reason for omission of the data. If no such information is available to the creator or if such information is not available in a well identified manner (e.g. buried in a free form narrative that contains additional information relevant to other sections) or if the creator requires that information be absent, the R2 section shall be entirely absent. (See section PCC TF-2: 5.3.4.2 for a list of appropriate statements).
- O
- An optional data element is one that may be provided, irrespective of whether the information is available or not. If the implementation elects to support this optional section, then its support shall meet the requirement set forth for the "Required if data present" or R2.
- C
- A conditional data element is one that is required, required if known or optional depending upon other conditions. These will have further notes explaining when the data element is required, et cetera.
| Note: | The definitions of R, R2, and O differ slightly from other IHE profiles. This is due in part to the fact that local regulations and policies may in fact prohibit the transmission of certain information, and that a human decision to transmit the information may be required in many cases. |
Folder Content Modules
This section contains modules that describe the content requirements of Folders used with XDS, XDM or XDR. When workflows are completed normally, the folders will contain documents with the optionality specified in the tables shown below. Under certain circumstances, the folders will not meet the optionality requirements described below, for example, when the patient leaves before treatment is completed.
HL7 Version 3.0 Content Modules
This section contains content modules based upon the HL7 CDA Release 2.0 Standard, and related standards and/or implementation guides.
CDA Document Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.1.1)Medical Documents Specification
CDA Header Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.2.5)PCC CDA Supplement 2:6.3.2.7 Authorization
CDA Section Content Modules
This list defines the sections that may appear in a medical document. It is intended to be a comprehensive list of all document sections that are used by any content profile defined in the Patient Care Coordination Technical Framework. All sections shall have a narrative component that may be freely formatted into normal text, lists, tables, or other appropriate human-readable presentations. Additional subsections or entry content modules may be required.
Impressions
- (1.3.6.1.4.1.19376.1.5.3.1.1.13.2.4)PCC CDA Supplement 2:6.3.3.9.7 Assessments
CDA and HL7 Version 3 Entry Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.4.1)PCC TF 2:6.3.4.3 Severity
- (1.3.6.1.4.1.19376.1.5.3.1.4.1.1)PCC TF 2:6.3.4.4 Problem Status Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.1.2)PCC TF 2:6.3.4.5 Health Status
- (1.3.6.1.4.1.19376.1.5.3.1.4.2)PCC TF 2:6.3.4.6 Comments
- (1.3.6.1.4.1.19376.1.5.3.1.4.3)PCC TF 2:6.3.4.7 Patient Medication Instructions
- (1.3.6.1.4.1.19376.1.5.3.1.4.3.1)PCC TF 2:6.3.4.8 Medication Fulfillment Instructions
- (1.3.6.1.4.1.19376.1.5.3.1.4.4)PCC TF 2:6.3.4.9 External References
- (1.3.6.1.4.1.19376.1.5.3.1.4.4.1)PCC TF 2:6.3.4.10 Internal References
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.1)PCC TF 2:6.3.4.11 Concern Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.2)PCC TF 2:6.3.4.12 Problem Concern Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.3)PCC TF 2:6.3.4.13 Allergy and Intolerance Concern
- (1.3.6.1.4.1.19376.1.5.3.1.4.5)PCC TF 2:6.3.4.14 Problem Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.6)PCC TF 2:6.3.4.15 Allergies and Intolerances
- (1.3.6.1.4.1.19376.1.5.3.1.4.7)PCC TF 2:6.3.4.16 Medications
- (1.3.6.1.4.1.19376.1.5.3.1.4.12)PCC TF 2:6.3.4.17 Immunizations
- (1.3.6.1.4.1.19376.1.5.3.1.4.7.3)PCC TF 2:6.3.4.18 Supply Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.7.2)PCC TF 2:6.3.4.19 Product Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.13)PCC TF 2:6.3.4.20 Simple Observations
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.1)PCC TF 2:6.3.4.21 Vital Signs Organizer
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.2)PCC TF 2:6.3.4.22 Vital Signs Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.15)PCC TF 2:6.3.4.23 Family History Organizer
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.4)PCC TF 2:6.3.4.24 Social History Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.3)PCC CDA Supplement 2:6.3.4.25 Family History Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.5)PCC CDA Supplement 2:6.3.4.26 Pregnancy Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.7)PCC CDA Supplement 2:6.3.4.29 Advance Directive Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.14)PCC CDA Supplement 2:6.3.4.31 Encounters
- (1.3.6.1.4.1.19376.1.5.3.1.4.19)PCC CDA Supplement 2:6.3.4.33 Procedure Entry
- (1.3.6.1.4.1.19376.1.5.3.1.1.12.3.1)PCC CDA Supplement 2:6.3.4.38 Pain Score Observation
HL7 Version 2 Content Modules
This section contains content modules based upon the HL7 Version 2 Standard, and related standards and/or implementation guides.
- (1.3.6.1.4.1.19376.1.5.3.1.1.19.1.1)Cancer Registry Pathology Report
PCC Value Sets
This section contains value sets used by Content Modules.
Appendix A - Examples Using PCC Content Profiles
Example documents conforming to each profile can be found on the IHE wiki at the following URLs.
| Profile and Content | URL |
|---|---|
| XDS-MS | |
| Referral Summary | XDSMS Example1 |
| Discharge Summary | XDSMS Example1 |
| XPHR | |
| XPHR Content | XPHR Example1 |
| XPHR Update | XPHR Example2 |
| (EDR) ED Referral | EDR Example |
| (APS) Antepartum Summary | APS Example |
| (EDES) | |
| Triage Note | EDES Example1 |
| ED Nursing Note | EDES Example2 |
| Composite Triage and Nursing Note | EDES Example3 |
| ED Physician Note | EDES Example4 |
| (FSA) Functional Status Section | FSA Example |
Appendix B - Validating CDA Documents using the Framework
Many of the constraints specified by the content modules defined in the PCC Technical Framework can be validated automatically by software. Automated validation is a very desirable capability, as it makes it easier for implementers to test the correctness of their implementations. With regard to validation of the content module, the PCC Technical Framework narrative is the authoritative specification, not any automated software tool. Having said that, it is still very easy to create a validation framework for the IHE PCC Technical Framework using a XML validation tool such as Schematron. Since each content module has a name (the template identifier), any XML instance that reports itself to be of that "class" can be validated by creating assertions that must be true for each constraint indicated for the content module. In the XML representation, the <templateId> element is a child of the element that is claiming conformance to the template named. Thus the general pattern of a Schematron that validates a specific template is shown below:
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReferralSummary'>
<rule context='*[cda:templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.1.3]"'>
<!-- one or more assertions made by the content module -->
</rule>
</pattern>
</schema>
Validating Documents
For document content modules, the pattern can be extended to support common document content module constraints as shown below:
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReferralSummary'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.1.3]"'>
<!-- Verify that the template id is used on the appropriate type of object -->
<assert test='../ClinicalDocument'>
Error: The referral content module can only be used on Clinical Documents.
</assert>
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.1.2"]'>
Error: The parent template identifier for medical summary is not present.
</assert>
<!-- Verify the document type code -->
<assert test='code[@code = "34133-9"]'>
Error: The document type code of a referral summary must be
34133-9 SUMMARIZATION OF EPISODE NOTE.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The document type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
<!-- Verify that all required data elements are present -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: A referral summary must contain a reason for referral.
</assert>
<!-- Alert on any missing required if known elements -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.8"]'>
Warning: A referral summary should contain a list of history of past illnesses.
</assert>
<!-- Note any missing optional elements -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.18"]'>
Note: This referral summary does not contain the pertinent review of systems.
</assert>
</rule>
</pattern>
</schema>
Validating Sections
The same pattern can be also applied to sections with just a few minor alterations.
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReasonForReferralUncoded'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<!-- Verify that the template id is used on the appropriate type of object -->
<assert test='section'>
Error: The coded reason for referral module can only be used on a section.
</assert>
<assert test='false'>
Manual: Manually verify that this section contains narrative providing the
reason for referral.
</assert>
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral
module is not present.
</assert>
<!-- Verify the section type code -->
<assert test='code[@code = "42349-1"]'>
Error: The section type code of the reason for referral section must be 42349-1
REASON FOR REFERRAL.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The section type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
</pattern>
<pattern name='ReasonForReferralCoded'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.2"]'>
<!-- The parent template will have already verified the type of object -->
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral
module is not present.
</assert>
<!-- Don't bother with the section type code, as the parent template caught it -->
<!-- Verify that all required data elements are present -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.4.13"]'>
Error: A coded reason for referral section must contain an simple observation.
</assert>
<!-- Alert on any missing required if known elements -->
<!-- Note any missing optional elements -->
</rule>
</pattern>
</schema>
A similar pattern can also be followed for Entry and Header content modules, and these are left as an exercise for the reader.
Phases of Validation and Types of Errors
Note that each message in the Schematrons shown above start with a simple text string that indicates whether the message indicates one of the following conditions:
- An error, e.g., the failure to transmit a required element,
- A warning, e.g., the failure to transmit a required if known element,
- A note, e.g., the failure to transmit an optional element.
- A manual test, e.g., a reminder to manually verify some piece of content.
Schematron supports the capability to group sets of rules into phases by the pattern name, and to specify which phases of validation should be run during processing. To take advantage of this capability, one simply breaks each <pattern> element above up into separate patterns depending upon whether the assertion indicates an error, warning, note or manual test, and then associate each pattern with a different phase. This is shown in the figure below.
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<phase id="errors">
<active pattern="ReasonForReferralUncoded_Errors"/>
<active pattern="ReasonForReferralCoded_Errors"/>
</phase>
<phase id="manual">
<active pattern="ReasonForReferralUncoded_Manual"/>
</phase>
<pattern name='ReasonForReferralUncoded_Errors'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<assert test='section'>
Error: The coded reason for referral module can only be used on a section.
</assert>
<assert test='code[@code = "42349-1"]'>
Error: The section type code of the reason for referral section must be 42349-1
REASON FOR REFERRAL.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The section type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
</rule>
</pattern>
<pattern name='ReasonForReferralUncoded_Manual'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<assert test='false'>
Manual: Manually verify that this section contains narrative providing the
reason for referral.
</assert>
</pattern>
<pattern name='ReasonForReferralCoded_Errors'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.2"]'>
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral not present.
</assert>
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.4.13"]'>
Error: A coded reason for referral section must contain an simple observation.
</assert>
</rule>
</pattern>
</schema>
Using these simple "templates" for template validation one can simply create a collection of Schematron patterns that can be used to validate the content modules in the PCC Technical Framework. Such Schematrons are expected to be made available as part of the MESA test tools that are provided to IHE Connectathon participants, and which will also be made available to the general public after connectathon.
Appendix C - Extensions to CDA Release 2.0
This section describes extensions to CDA Release 2.0 that are used by the IHE Patient Care Coordination Technical Framework.
IHE PCC Extensions
All Extensions to CDA Release 2.0 created by the IHE PCC Technical Committee are in the namespace urn:ihe:pcc:hl7v3.
The approach used to create extension elements created for the PCC Technical Framework is the same as was used for the HL7 Care Record Summary (see Appendix E) and the ASTM/HL7 Continuity of Care Document (see secion 7.2).
replacementOf
The <replacementOf> extension element is applied to a section appearing in a PHR Update Document to indicate that that section's content should replace that of a previously existing section. The identifier of the previously existing section is given so that the PHR Manager receiving the Update content will know which section to replace. The model for this extension is shown below.
Use of this extension is shown below. The <replacementOf> element appears after all other elements within the <section> element. The <id> element appearing in the <externalDocumentSection> element shall provide the identifier of the section being replaced in the parent document.
<section>
<id root=' ' extension=' '/>
|
Extensions Defined Elsewhere used by IHE PCC
Entity Identifiers
There is often a need to record an identifer for an entity so that it can be subsequently referenced. This extension provides a mechnism to store that identifier. The element appears after any <realm>, <typeId> or <templateId> elements, but before all others in the entity where it is used:
<playingEntity classCode='ENT' determinerCode='INSTANCE'> <sdtc:id root='1.3.6.4.1.4.1.2835.2' extension='EntityID'/> : . </playingEntity>
Patient Identifier
There is a need to record the identifer by which a patient is known to another healthcare provider. This extension provides a role link between the assigned, related or associated entity, and the patient role.
Use of this extension to record the identifier under which the patient is known to a provider is shown below.
<assignedEntity>
<id extension='1' root='1.3.6.4.1.4.1.2835.1'/>
|
The <patient> element records the link between the related, assigned or associated entity and the patient. The <id> element provides the identifier for the patient. The root attribute of the <id> should be the namespace used for patient identifiers by the entity. The extension attribute of the <id> element shall be the patient's medical record number or other identifier used by the entity to identify the patient.


