Specimen Event Tracking
Specimen Event Tracking (SET) profile is a project of the IHE PaLM Technical committee that was started in 2016. The purpose of this new profile of the PaLM domain is to define a series of use cases and all related events relevant to be tracked, when one or more macro operations involving a specimen occur (i.e., container preparation, collection, movement from one facility to another one, archiving, and so on).
Summary
The Specimen Event Tracking (SET) profile covers use cases and transactions related to the tracking of biological specimens in vitro collected for the purpose of diagnostic testing, during their entire lifecycle, from creation to storage inside a laboratory specimen archive or a biobank for future usage, and to final disposal. Specimen workflows can involve a ward and a laboratory in the same hospital, different laboratory facilities inside the same institution, or across different institutions. In the latter case, specimens need to be transferred by a courier service from the sending institution to the receiving institution. Another important use case to be tracked is the specimen creation for the specific purpose of becoming part of a biobank for a research institution or program. The SET profile tracks macro activities related to specimens, such as collecting, shipping, receiving, accepting... Micro operations part of a macro activity (e.g. decapping a tube) are out of scope of the profile. For most recent information on the SET please see: http://wiki.ihe.net/index.php/Pathology_and_Laboratory_Medicine_(PaLM)#Current_Activity.
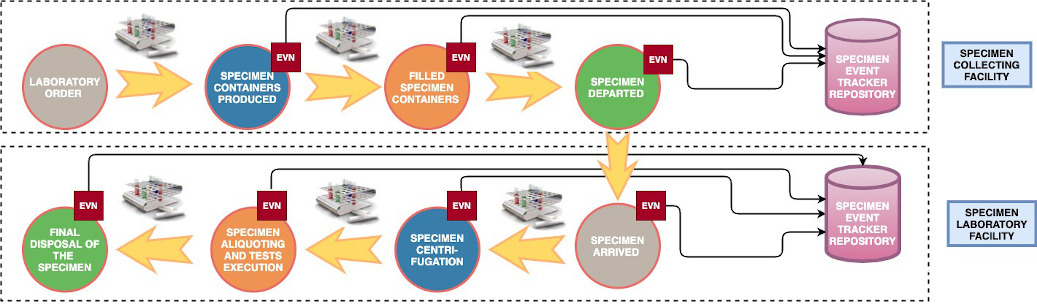
Figure 1 - Some of the main events that might occur to a Specimen during its overall lifecycle, and that the SET profile aims to track. Traceability messages are collected at each step by an Informer entity and sent to a tracker entity (repository). Notice that, according to the use case, the trackers may be more than one, if the Collecting and Laboratory, if the Collecting Facility and the Laboratory have independent tracking environments.
Benefits
- Keep under control the chain-of-custody of the specimen, when operation addressed by all the other profiles occur to the specimen
- Prevent specimen loss, opportunity to raise alerts if some exceptions occurs to the process (i.e., broken container, specimen collection unavailable)
- All events might be collected in a single place and use to improve the overall process
Details
<A few paragraphs, if appropriate, providing more details (mostly in user-speak, not tech-speak) on what the profile does and how it works.>
<If the user might be familiar with the mechanisms used by the profile, you can mention them here. E.g. Evidence Documents is based on DICOM Structured Report (SR) Templates.>
<If the user might have an appreciation for the problems addressed in the profile, you can mention them here, but keep it short. E.g. Mapping HL7 Order fields to DICOM Modality Worklist attributes can be inconsistent in the marketplace, so Scheduled Workflow provides vendors with more detailed instructions.>
Systems Affected
<List (in user terms) the types of systems they might expect to have implemented actors from this profile, e.g. RIS, PACS, HIS, CAD Workstation, etc. and for each, how it would participate.>
- EHR-S
- LIS
- Lab automation manager
- ...
<Insert an actor-transaction diagram, and or list of Content Definitions>
Specification
Profile Status: Final Text <Replace "Final Text" with "Trial Implementation" or "Public Comment" as appropriate.>
Documents: Link to current draft document: [1] Link to SET matrix with collection of all data elements per use case: https://wiki.ihe.net/index.php/File:2019_01_SET_new_mapping_structure_pre_HL7_call.xlsx
Underlying Standards:
- HL7 v2 - chapters 7 (for OSM^R26) and 13 (for SSU^U03) HL7
- ...
See Also
<The following sections can be left out if there is nothing to point to. This is just to show where such information can go.>
Related Profiles
<List profiles this one depends on, profiles that depend on this one, profiles that are synergistic with this one. Start with the name of the other profile as a link and then explain the relationship.>
- <to be updatedReporting Workflow [RWF] may use Evidence Documents as inputs to the reporting process.>
Consumer Information
The Profile FAQ Template answers typical questions about what the Profile does. <Replace the link with a link to the actual FAQ page for the Profile>
The Profile Purchasing Template describes considerations when purchasing equipment to deploy this Profile. <Replace the link with a link to the actual Purchasing page for the Profile>
Implementer Information
The Profile Implementation Template provides additional information about implementing this Profile in software. <Replace the link with a link to the actual Implementation page for the Profile>
Reference Articles
<List References (good and bad) (with link if possible) to Journal Articles that mention IHE's work (and hopefully include some analysis). Go ahead, Google: IHE <Profile Name> abstract or Google: IHE <Profile Name> and under the "more" select "Scholar". You might be surprised. >
This page is based on the Profile Overview Template