PCD Implantable Device Cardiac Observation
Implantable Device - Cardiac - Observation (IDCO) communicates IDC / "pacemaker" information to enterprise applications such as EHRs.
Summary
This profile describes a means to transfer information from an interrogated implantable cardiac device to information management systems. This profile is named Implantable Device – Cardiac – Observation or IDCO for short.
Benefits
- Standards based translation and transfer of summary device interrogation information
- Improved workflow efficiencies in Cardiology and Electrophysiology practices from management of “key” summary implantable rhythm control device interrogation information in a central system such as an electronic health record system (EHR) or a device clinic management system.
Details
Cardiac physicians follow patients with implantable cardiac devices from multiple vendors. These devices are categorized as pacemakers, implantable cardioverter defibrillators, cardiac resynchronization therapy devices, and cardiac monitor devices.
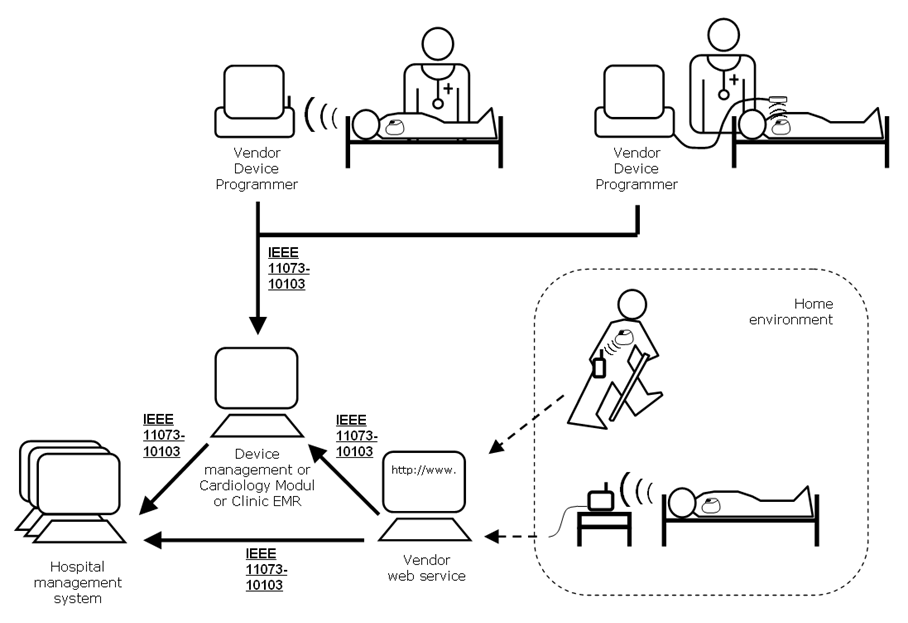
As part of patient follow-up an interrogation of a cardiac device is performed (either in-clinic or from a remote location). Information is collected about the device such as device identification, therapy settings, device diagnostics, and device testing. These interrogations are performed by vendor proprietary equipment.
To improve workflow efficiencies Cardiology and Electrophysiology practices require the management of “key” summary implantable rhythm control device interrogation information in a central system such as an electronic health record system (EHR) or a device clinic management system. To address this requirement, the Implantable Device – Cardiac – Observation (IDCO) Profile defines a standards based translation and transfer of summary device interrogation information from the interrogation system to the information management system.
With the increased use of EHR systems, the networking of in-clinic and remote follow-up systems for implanted cardiac devices, and the continued growth of the implantable cardiac device market, the importance of this profile to clinicians has become more acute. Strong device and EHR vendor participation in the IDCO profile development is an acknowledgement of this importance. As the IDCO Profile and associated standards continue to evolve there is little doubt that the use of this profile will be a critical component of comprehensive cardiac care.
The Device Observation Reporter (DOR) actor receives data from IDCs and maps the received data to transactions providing consistent syntax and semantics.
The Device Observation Consumer (DOC) is the actor responsible for receiving IDC data from the Device Observation Reporter.
Specification
Profile Status: Final Text
Documents:
- The Patient Care Devices Technical Framework is the official master document for this Profile. The specification of the PCD-09 message format can be found in Volume 2.
Underlying Standards:
- HL7
- ISO/IEEE 11073-10101 (Point-Of-Care Medical Device Communication - Part 10101: Nomenclature)
- ISO/IEEE 11073-10103 (Point-Of-Care Medical Device Communication - Part 10103: Nomenclature - Implantable device, cardiac)
Systems Affected
- EHR systems may store, manage, and/or display IDCO reports.