PCC TF-1/APR
Volume 1
HIMSS and RSNA
Integrating the Healthcare Enterprise
IHE Patient Care Coordination
Antepartum Record (APR)
Technical Framework Supplement
Volume I
Revision 3.0
2008-2009
Public Comment
- (PCC TF-1/Preface)Preface to Volume I of the PCC Technical Framework
- (PCC TF-1/Introduction)Introduction to the PCC Technical Framework
- (PCC TF-1/About)About the Patient Care Coordination Integration Profiles
- (PCC TF-1/Dependencies)Dependencies of the PCC Integration Profiles
- (PCC TF-1/Overview)PCC Integration Profiles Overview
- (PCC TF-1/History)History of Annual Changes
- (PCC TF-1/Product Implementations)Product Implementations
Introduction
This is a draft of the Antepartum Record Profile (APR) supplement to the PCC Technical Framework. This draft is a work in progress, not the official supplement or profile.
Profile Abstract
The Antepartum Record Profile (APR) is based on data elements from prenatal records currently in common use, and includes the following documents:
- Antepartum History & Physical – The initial assessment and physical
- Antepartum Summary – Summary of the most critical information
- Antepartum Laboratory – Laboratory Evaluations
- Antepartum Education – Education Record
Additional commonly used forms not included in this profile are:
- A patient generated obstetric medical history
- A postpartum form
A sample form showing the data elements may be found at: http://www.acog.org/acb-custom/aa128.pdf
This profile defines the implementation of HL7 CDA documents to represent these data elements along with the XDS, XDR and XDM bindings. This profile also defines mechanisms to group them into a single logical folder.
Glossary
The following elements are found in the Antepartum History & Physical document of the Antepartum Record:
- Abortion, Induced (AB, Induced)
- Number of induced abortions by patient. An induced abortion is a deliberate termination of pregnancy.
- Abortion Spontaneous (AB, Spontaneous)
- Number of spontaneous abortions by patient. A spontaneous abortion is a natural loss of the products of conception.
- Ectopic pregnancy
- Number of ectopic pregnancies by patient. An ectopic pregnancy is the development of a fertilized ovum outside the uterus, as in a Fallopian tube.
- Estimated Date of Delivery(EDD)/Estimated Date of Confinement(EDC)
- Date of anticipated delivery (confinement).
- Final/Corrected Estimated Date of Delivery (EDD)
- Corrected EDD/EDC based upon parameters such as ultrasound, first auscultation of fetal heart tones, etc.
- Full term
- Number of babies the mother has delivered that were between 37 and 42 completed weeks of gestation.
- Living Children
- Number of living children of patient
- Multiple births
- Number of deliveries of more than one baby by patient
- Premature
- Delivery between 20 and 36 6/7 weeks gestation
- Stillbirth
- An infant delivered without signs of life after reaching mid-second trimester to full term gestational age. In the US this is usually after 20 or greater weeks gestation. In the UK this has been reported as an infant delivered without signs of life until after 24 weeks gestation.
- Total Pregnancies
- Number of total pregnancies
Antepartum History & Physical - Menstrual History
- Birth Control Pills (BCP)
- Oral contraceptives.
- Frequency
- Duration of the monthly menstrual cycle; from first day of menses to the first day of next menses.
- hCG+
- Human Chorionic Gonadotropin pregnancy test.
- LMP (last menstrual period)
- Date measured as the first day of the patient's most recent menstrual period.
- Approximate (month known) - Patient is unsure of exact date but can offer an approximate date.
- Definite - Patient can say with certainty the date of her last menstrual period.
- Final - Finally agreed upon date of last menstrual period.
- Unknown - Patient does not know the date of her last menstrual period.
- Menarche
- Age at onset of initial menstrual period.
- Menses Monthly
- Menses is the monthly flow of blood and cellular debris from the uterus that begins at puberty and ceases at menopause.
- Normal Amount/duration
- Last menstrual was typical in amount and duration.
- Prior Menses
- Date of most recent menstrual period.
Antepartum History & Physical - Past Pregnancies
- Anesthesia
- The loss of the ability to feel pain caused by administration of a drug or other intervention.
- Artificial Reproductive Technology (ART) Treatment
- Fertility procedures in which both eggs and sperm are handled in the laboratory (in vitro) to establish a pregnancy.
- Autoimmune disorder
- An autoimmune disorder is a condition in which the body attacks its own tissues. (ACOG)
- Birth weight
- Weight of infant at birth.
- Delivery Date
- Date of delivery of patient's previous pregnancies.
- DES
- Diethylstilbesterol
- D (Rh) sensitized
- Rh negative mother is sensitized to the Rh D antigen. A sensitized mother produces IgG anti-D (antibody) that crosses the placenta and coats D-positive fetal red cells which are then destroyed in the fetal spleen.
- Gestational Age weeks
- The number of weeks elapsed between the first day of the last normal menstrual period and the date of delivery.
- Infertility
- Infertility primarily refers to the biological inability of a man or a woman to contribute to conception. Infertility may also refer to the state of a woman who is unable to carry a pregnancy to full term.
- Kidney disease
- Kidney disease is either a declining or a sudden loss in renal function.
- Length of labor
- The interval between onset of contractions and childbirth.
- Place of Delivery
- Hospital name, city and state if known.
- Preterm labor
- Labor that begins before 37 weeks gestation.
- Pulmonary (TB, Asthma)
- Diseases or disorders of the lungs, i.e. asthma, tuberculosis or other pulmonary problems.
- Sex Male/Female
- Sex of patient's previously delivered babies.
- Type Delivery
- Type of delivery in pregnancy: Vaginal (spontaneous, forceps, vacuum), Cesarean section (low-transverse, classical, low-vertical).
- Urinary Tract Infection (UTI)
- A urinary tract infection (UTI) is a bacterial infection that affects any part of the urinary tract.
- Uterine Anomaly
- – Any uterine structural abnormalities.
- Varicosities/Phlebitis
- Swelling or inflammation of veins.
Antepartum History & Physical - Other elements:
- Abdomen
- Area of the body that lies between the chest and the pelvis and encloses the stomach, intestines, liver, spleen and pancreas
- Adnexa
- Appendages of the uterus which include the fallopian tubes, the ovaries and the supporting ligaments of the uterus.
- BMI - Body Mass Index.
- Measurement of the relative percentages of fat and muscle mass in the human body.
- BP - Blood Pressure
- Pressure exerted by the blood against the walls of the arteries, maintained by the contraction of the left ventricle, the resistance of the arterioles and capillaries, the elasticity of the arterial walls, and by the viscosity and volume of the blood.
- Breasts
- In humans, one of the paired regions in the anterior portion of the thorax. The breasts consists of mammary glands, the skin, the muscles, the adipose tissue and connective tissues.
- Cervix
- The lower, narrow end of the uterus, which protrudes into the vagina. (ACOG)
- Diagonal Conjugate
- The distance from the promontory of the sacrum to the lower margin of the pubic symphysis
- Extremities
- A bodily limb or appendage.
- Fundus
- The fundus of the uterus is the top portion of the uterus, opposite from the cervix. Fundal height, measured from the top of the pubic bone, is routinely measured in pregnancy to determine growth rates.
- Gynecoid pelvic type
- The normal female pelvis.
- Heart
- The hollow, muscular organ that maintains the circulation of the blood.
- HEENT
- Head, Eyes, Ears, Nose and Throat
- Height
- Measurement of stature
- Lungs
- Either of the pair of organs occupying the cavity of the thorax that effect the aeration of the blood.
- Lymph nodes
- Any of the accumulations of lymphoid tissue organized as definite lymphoid organs varying from 1 to 25 mm in diameter situated along the course of lymphatic vessels and consisting of an outer cortical and inner medullary part.
- Rectum
- The distal segment of the large intestine, between the sigmoid colon and the anal canal.
- Sacrum
- Triangular bone below the lumbar vertebrae.
- Skin
- Outer protective covering of the body
- Spines
- (Ischial Spines) Two parts of the maternal pelvis resulting from the bony processes projecting backward and medially from the posterior border of the ischium.
- Subpubic arch
- Arch formed by the conjoined rami of the ischia and pubic bones of the two sides of the body.
- Teeth
- one of the hard, calcified structures set in the alveloar processes of the jaws for the biting and mastication of food.
- Thyroid
- The thyroid gland. One of the largest endocrine glands in the body. This gland is found in the neck below the thyroid cartilage and at approximately the same level as the cricoid cartilage. The thyroid controls how quickly the body burns energy, makes proteins, and how sensitive the body should be to other hormones.
- Uterus size
- In pregnancy the uterine size is estimated in terms of weeks of gestation. e.g 12 weeks if the fundus reaches the top of the smphysis pubis or 20 weeks' gestation when the fundus reaches the umbilicus.
- Vagina
- The genital canal in the female, leading from the opening of the vulva to the cervix of the uterus.
- Vulva
- The external genital organs of the female, including the labia majora, labia minora, clitoris, and vestibule of the vagina.
- Patient Weight
- A measurement of mass.
The following terms are found in the Antepartum Laboratory document of the Antepartum Record:
- 1st Trimester Aneuploidy risk assessment (Free or Total)
- Non-invasive screening for chromosomal abnormalities, such as Down syndrome, performed in the first trimester. Screening tests that uses a combination of fetal measurements (crown rump length and nuchal translucency) and maternal blood tests for beta-human chorionic gonadotropin (hCG) and pregnancy associated plasma protein (PAPP-A) to determine risk for trisomy 21, trisomy 13 and trisomy 18.
- 2nd Trimester serum screening
- Non-invasive screening test for chromosomal abnormalities, such as Down yndrome, trisomy 18, or open neural defects. Blood test to measure alpha-fetoprotein (AFP), estriol, human chorionic gonadotropin (hCG) [free or total], and inhibin-A.
- Amniocentesis (Amnio)
- Percutaneous transabdominal puncture of the uterus during pregnancy to obtain amniotic fluid.
- Amniotic Fluid (AFP) Test
- A test to detect the presence of Alpha-fetoprotein in amniotic fluid.
- Antibody screen
- A blood test to detect antibodies against red blood cell antigens.
- Anti-D Immune Globulin (RHIG)
- Anti-D antibodies given to prevent sensitization to the RhD antigen on red blood cells.
- Blood type
- Test to determine blood group, i.e. A, B, AB or O
- Chlamydia Test
- Test done to detect the bacterium, Chlamydia trachomatis.
- Cystic Fibrosis Screening Test
- Test to detect gene mutations that cause cystic fibrosis.
- Chorionic Villi Sampling (CVS)
- A method of sampling the cells of the placental chorionic villi, done either transabdominally or transcervically.
- D (Rh) Antibody screen
- A blood screening test for presence of IgG antibodies to the Rh D antigen on red blood cells.
- D (Rh) type
- A blood test to detect the presence of the Rh D red blood surface antigen.
- Diabetes screen
- Laboratory test to screen for gestational diabetes.
- Familial Dysautonomia
- Autosomal disorder of the peripheral and autonomic nervous systems limited to individuals of Ashkenazic Jewish descent; clinical manifestations are present at birth and include diminished lacrimation, defective thermoregulation, orthostatic hypotension, fixed pupils, excessive sweating, loss of pain and temperature sensation, and absent reflexes; pathologic features include reduced numbers of small diameter peripheral nerve fibers and autonomic ganglion neurons.
- Genetic Screening Test
- Screening for genetic disorders, e.g. sickle cell, Thalassemia, Tay-Sachs, Canavan, cystic fibrosis, fragile X syndrome, or Duchenne’s muscular dystrophy.
- Gonorrhea Test
- Test to detect Neisseria gonorrhea
- Group B Streptococcus Rectovaginal Culture (Group B Strep)
- A test to determine the presence of group B streptococcus (streptococcus agalactiae) in the lower genital tract in pregnant women.
- GTT (if screen abnormal)
- Glucose Tolerance Test. Used to determine how quickly the body metabolizes blood sugar. Test to diagnose gestational diabetes mellitus.
- HBsAg Test
- Test for the detection of the surface antigen of the Hepatitis-B virus.
- HCT/HGB/MCV
- HCT- Hematocrit – A blood test measuring the percentage of red blood cells found in a given volume of whole blood.
- HGB- Hemoglobin – A blood test measuring the level of the protein carrying oxygen in red blood cells.
- MCV - Mean corpuscular volume - The average volume of red blood cells calculated from the hematocrit red blood cell count
- Hemoglobin Electrophoresis
- A blood test done to measure the different types of hemoglobin. The test can detect abnormal levels of hemoglobin such as that found in sickle cell anemia.
- HIV Test
- A test to detect for the presence of antibodies to the human immunodeficiency virus.
- HIV Counseling
- Discussion with pregnant patient regarding Human Immunodeficiency Virus/ HIV status, risks and prevention strategies.
- Karotype
- Test done on cells/tissue to identify and evaluate the number, shape, and size of chromosomes.
- MSAFP - Maternal Serum Alpha-Fetoprotein
- A screening blood serum test on the mother for to determine the level of alpha-fetoprotein.
- Multiple marker screening test
- A maternal blood serum screening test for the detection of Down syndrome, trisomy 18, and neural tube defects in the fetus. The following analytes are measured: alpha-fetoprotein, human chorionic gonadotriopin, estriol, and inhibin-A. When the first three analytes are used, this is also called a maternal serum triple screen or a maternal serum quad screen when all four analytes are used.
- Pap test
- Cervical cytology test to determine abnormal cells of the cervix.
- PPD Skin Test
- - Mantoux test with purified protein derivative to screen for exposure to tuberculosis.
- Rubella Test
- A blood test to detect the presence of antibodies against the rubella virus (German measles).
- Tay-Sachs Screening Test
- A blood test done to measure the amount of beta-hexosaminidase A or B activity in serum or white blood cells, or for the most common DNA mutations causing Tay Sachs disease.
- Ultrasound
- A radiologic study using sound waves used in the assessment of gestational age, size, growth, anatomy, and blood flow of a fetus or in the assessment of maternal anatomy and blood flow.
- Urine Culture
- A Test that it used to detect the presence of bacteria or other organism in the urine.
- Urine Screen
- A physical, chemical, and / or microscopic examination of the urine. It may be used to screen for / or to detect abnormal kidney function, kidney stones, urinary tract infections, or substance abuse.
- Varicella
- A blood test to detect the presence of anti-varicella antibodies.
- VDRL (Venereal Disease Research Laboratories)
- A blood test to screen for the presence of antibodies against Treponema pallidum, the bacteria that causes syphilis.
The following terms are found in the Antepartum Education document of the Antepartum Record:
First Trimester
- Alcohol
- Discussion with patient about past and present use of alcohol and the perinatal implications of continued use during pregnancy; referral to treatment program if appropriate.
- Anticipated Course of prenatal care
- Discussion with the patient on the scope of care that will be performed in the office, lab work that may be performed, signs and symptoms that should be reported, anticipated schedule of visits, physician coverage of labor and delivery.
- Childbirth classes/hospital facilities
- Discussion with the patient on educational programs available for childbirth and hospital choice.
- Domestic violence
- Screening/Discussion with patient regarding physical threats/abuse/safety concerns; referral to appropriate counseling, legal and/or social advocacy program if appropriate.
- Environmental/Work hazards
- Discussion with patient about potential exposures to environmental agents at work, home, or locations that may affect pregnancy.
- Exercise
- Discussion with patient on appropriate level of exercise activities during the pregnancy.
- Illicit/Recreational drugs
- Discussion with patient about past and present use of illicit or recreational drugs and the perinatal implications of continued use during pregnancy; referral to treatment program if appropriate.
- Indications for ultrasounds
- Discussion with patient regarding reasons ultrasound test will be performed during pregnancy.
- Influenza vaccine
- Discussion with patient of risks/benefits of influenza and influenza vaccine during pregnancy.
- Nutrition and weight gain counseling, special diet
- Information about balanced nutrition, ideal caloric intake and weight gain.
- Risk factors identified by prenatal history
- Seatbelt use
- Discussion with patient on use of seatbelts.
- Sexual activity
- Discussion with the patient of sexual activity: concerns, restrictions, warning signs and/or safe sex practices.
- Smoking counseling
- Discussion with patient regarding smoking cessation and smoke exposure.
- Tobacco (Ask,advise,assess,assist,and arrange)
- status; Advise patient to stop smoking; Assess patient's willingness to attempt to quit smoking; Assist patients who are interested in quitting by providing pregnancy specific cessation materials; Arrange follow up visits to track progress.
- Toxoplasmosis precautions
- Discussion with patient of risk factors for toxoplasmosis and precautions for avoiding/preventing infection.
- Travel
- Discussion with patient on travel precautions, if any.
- Use of any medications (including supplements, vitamins, herbs or OTC drugs)
- Discussion with patient of risks/benefits/safety of any medications currently used by patient.
Second Trimester
- Abnormal lab values
- Discussion with patient of lab results that fall outside normal range and that may require further testing.
- Domestic violence
- Screening/Discussion with patient regarding physical threats/abuse/safety concerns; referral to appropriate counseling, legal and/or social advocacy program if appropriate.
- Influenza vaccine
- Discussion with patient of risks/benefits of influenza and influenza vaccine.
- Postpartum family planning/tubal sterilization
- Discussion with patient of intended postpartum contraception options, including tubal sterilization.
- Selecting a newborn care provider
- Discussion with patient to identify newborn care provider; referral to resources to help patient choose provider if none previously identified.
- Signs and symptoms of preterm labor
- Discussion with patient on risks, signs and symptoms of preterm labor.
- Smoking counseling
- Discussion with patient regarding smoking cessation and smoke exposure.
Third Trimester
- Anesthesia/Analgesia plans
- Discussion with patient to determine intended method of pain management/discomfort during labor and delivery.
- Breast or bottle feeding
- Discussion with patient of nutritional advantages/disadvantages of human breast milk, bottled formula; advise on available lactation consultation services.
- Circumcision
- Discussion with patient on circumcision of male newborn.
- Domestic violence
- Screening/Discussion with patient regarding physical threats/abuse/safety concerns; referral to appropriate counseling, legal and/or social advocacy program if appropriate.
- Family medical leave or disability forms
- Discussion with patient about any forms the patient will need completed for employment or insurance purposes.
- Fetal Movement monitoring
- Discussion with patient regarding her perception and assessment of fetal movement.
- Influenza vaccine
- Discussion with patient of risks/benefits of influenza and influenza vaccine.
- Labor signs
- Discussion with patient on signs of labor, i.e. contractions, membrane rupture, bleeding, etc.
- Newborn education (Newborn screening, jaundice, SIDS, car seat)
- Prenatal discussion with patient of preventive public health screening procedures available to newborns; testing that will occur on baby after birth to screen for up to 30 disorders. Additional items may include infants risk for developing jaundice. Discussion of positioning of infant to reduce SIDS risk. Education regarding car seat safety.
- Postpartum depression
- Discussion with patient of signs of postpartum depression.
- Postterm counseling
- Discussion with patient of risks of pregnancy extending beyond 42 weeks.
- Signs & Symptoms of Pregnancy-induced hypertension
- Discussion with patient of signs and symptoms of hypertension.
- Smoking counseling
- Discussion with patient regarding smoking cessation and smoke exposure.
- VBAC (Vaginal Birth After Cesarean) counseling
- Discussion with patient of risks/benefits of vaginal birth after previous cesarean surgery.
- History and physical have been sent to hospital
- Notation of date and initials of person transmitting history and physical to hospital prior to delivery.
- Tubal sterilization consent signed
- Notation of date the consent form for tubal sterilization signed and the initials of person witnessing.
Open Issues
- How does the XDS Folder structure need to be handled?
- Several LOINC and SNOMED codes are in the process of being created. These codes are denoted by a preceeding "xx-" or "XX-" with an abbreviated description of the code following.
Closed Issues
- For Antepartum Laboratory there is a LOINC code for Laboratory Studies (26436-6) - is this too general? Should a new code be requested specific to Antepartum labs? The concern is that this could cause mapping issues in an EMR that has other lab results that are considered to be specific to antepartum that would live under that same loinc section code. the IHE formatCode supplied in the XDS Metadata will identify this as an Antepartum Laboratory document so a LOINC code is not needed.
Volume I
Add the following bullet to the list of profiles
- Antepartum Record - A folder of content profiles that contains the record of antepartum care including initial patient history and physical, recurring evaluations of mother and fetus(es), laboratory studies, patient education, and on-going plans of care.
Dependencies
Add the following row(s) to the list of dependencies
| Integration Profile | Dependency | Dependency Type | Purpose |
|---|---|---|---|
| Antepartum Record | Sharing of Laboratory Reports (XD-LAB) | child | share laboratory results |
Antepartum Record (APR)
The Antepartum Record Profile (APR) is based on the data elements from prenatal records currently in common use.
Obstetric patients in labor and admitted to the hospital or birthing facility optimally would have a complete summary of their antepartum ambulatory care available at the time of admission to evaluate and / or ameliorate risk. This same data is required at any visit to the birthing facility or hospital for any other problems or special care needs of the patient. The antepartum record optimally would be available in its entirety for appropriate continuity of care and legal concerns.
The aggregated record provides important information for all health care professionals who are part of the patient's obstetric care team. Patients may incorporate the data from this aggregated record into their personal health record. Administration staff may use data for billing and payment purposes.
A typical pregnancy duration is approximately 40 weeks. Patient care during that time includes an initial history and physical examination, followed by repetitive office visits with multiple laboratory studies, imaging/ ultrasound studies, and serial physical examinations. As the patient is seen over a finite period for care, aggregation of data relevant to the evaluation of the obstetric patient upon presentation to the birthing facility or hospital is commonly collected on paper forms. This antepartum record contains the most critical information needed to provide care for the patient during pregnancy, delivery and the post-partum period. This data must all be presented and evaluated upon entry to the birthing facility or hospital to ensure optimal continuity of care for the patient and the fetus.
Although the patient and her care provider may plan for a vaginal method of delivery, there is a substantive chance the delivery route may be surgical, requiring anesthesia and post-surgical care.
Current practice is to copy the patient's paper chart at various times during the pregnancy (as at 28 weeks and at 36 weeks of completed gestation), and transport the copies of the chart to the hospital the patient intends to use for delivery. Should the patient arrive at the birthing facility or hospital prior to the chart copy arriving, or if the chart (or information within the chart) is missing on presentation of the patient (a frequent occurrence), the care team must repeat laboratory or imaging studies. This results in unwarranted and duplicative tests, is wasteful of time and resources, and leads to dissatisfied patients. Further, missing or incomplete information about the patient’s clinical status may create a situation where critical information is unavailable which may ultimately result in an injury, inadequate aftercare, or other undesirable outcomes.
A large portion of patients arrive at the birthing facility or hospital without complete documentation. In one recent U.S. study, approximately 70 % of patients (with paper charts) arrived at the birthing facility without their current medical record being available. While only one hospital was involved in this study, one can see the extent of the issue, with pregnant patients possibly going to a different hospital than planned (preterm labor, rapid labor and unable to make it to the planned delivery hospital, or visiting a distant city), moving mid-care, or with a covering physician (rather than the primary obstetrician) on call.
In a Swedish study done in the 1990’s, critical data on paper records were incomplete from 45 to 87.5% of the time. Thus, availability of current medical records remains a significant problem for most hospital birthing facilities; availability of key information electronically will significantly enhance patient safety.
Use Cases
Use Case 1: Basic Antepartum Record Use Case
This use case reflects the course of care during an uncomplicated pregnancy.
Pre-condition
The patient’s obstetrician sees the patient for her initial and subsequent prenatal visits. During the initial and/or subsequent prenatal visits information is collected and may be updated within the office Electronic Health Record (EHR), these include:
- Patient demographics
- Menstrual history
- Obstetric history
- Medical history including surgical history, psych-social history
- Genetic history and screening/Teratology counseling
- Infection history
- Family history
- Initial and subsequent physical examination
- Medications
- Problems and risk factors for preterm birth
- Allergies
- Prenatal visit information
- Prenatal Laboratory results
- Documentation of patient education and counseling
- Plans for care
The information collected during the patient’s prenatal visits make up the components which are included in the patient’s Antepartum Record.
Event(s)
Scenario 1 - At a specified time an initial and/or subsequent patient Antepartum Record is transmitted by the patient’s prenatal care provider EHR to the intended facility for delivery.
The intended facility of delivery health information system receives the transmitted initial and/or subsequent patient Antepartum Record.
Scenario 2 - At a specified time the initial and/or subsequent patient Antepartum Record registry information is transmitted by the patient’s obstetrician EHR to a registry.
The facility of delivery health information system queries the registry repository for the applicable patient’s Antepartum Record(s). A request is made for the patient’s Antepartum Record. The applicable system which contains the patient’s Antepartum Record then makes available the patients Antepartum Record information to the requesting facility of delivery.
Post-condition
The received patient Antepartum Record can be viewed and/or imported into the facility for delivery health information system to facilitate patient care by healthcare professional at the time of delivery for the mother and newborn.
Use Case 2: Antepartum Consultative Care
This use case reflects an example of perinatologist consultative prenatal care.
Pre-condition
The patient’s prenatal care provider sees the patient for her pregnancy in the ambulatory (office) setting. During the pregnancy, the patient is noted to have a medical problem requiring consultation with a maternal-fetal medicine specialist (perinatologist). The office obtains pre-authorization from the insurance payer for the consult and for the intended or anticipated route of delivery. Preauthorization information is transmitted to both the consultant and to the hospital.
Events
The patient is seen in the prenatal care provider’s office where a complete health history (e.g. medical and surgical history, psycho-social history) is obtained and recorded in the office EHR and sent to the consultive care provider office EHR. Data from the perinatologist’s consultation report is incorporated as appropriate. Laboratory and imaging reports ordered by the perinatologist consultant as well as the perinatologist’s consultation report are displayed electronically to the prenatal care provider. The prenatal care provider reviews the consultation report from the perinatologist’s office and imaging studies ordered by the perinatologist along with current recorded data. Physical exam reveals some abnormalities. The prenatal care provider orders additional laboratory studies, and sends the patient to the hospital or birthing facility.
When the laboratory results return, the prenatal care provider completes the admission history and physical, allergies, medications, includes the data prepared or ordered by the perinatologist, and makes it available to the hospital or birthing facility. This data includes an assessment of the patient’s health status, and the requisite data summarized from the antepartum care given. The care team assures the complete collection of documents needed is available and that there is a suitable environment with appropriate support for post-delivery after-care.
Post-condition
The pre-delivery history and physical and Antepartum Summary with appropriate relationships to the perinatologist consultation, and all the antepartum laboratory and imaging studies are available to the obstetrician and the hospital or birthing center personnel for incorporation into their respective EHRs. The history and physical is also available to the patient for viewing and incorporation into the patient’s PHR, and into the newborn baby’s PHR.
Use Case 3: Antepartum Collaborative Care
This use case reflects two-way transmission of data in an example of collaborative care.
Pre-condition
A pregnant diabetic patient is seen by her prenatal care provider in the office for prenatal care. An ultrasound is performed to determine gestational age. The patient is sent to a consultant (e.g. perinatologist) as a high-risk patient. Her prenatal care provider transmits preauthorization insurance information, labs and anticipated route of delivery to the consultant and/or hospital birthing facility.
Events
The patient returns to her consultant biweekly for blood testing and ultrasounds (when necessary) in addition to regular ob visits. The consultant reports back to the obstetrician after each visit. Complete history and physical, imaging and additional labs are performed during patient’s regular visit with her prenatal care provider.
The patient arrives at birthing facility. Prenatal care provider completes the admission history and physical, allergies, medications, and includes the data prepared or ordered by the consultant, and makes it available to the hospital birthing facility. This data includes an assessment of the patient’s health status, and the requisite data summarized from the antepartum care given. The care team documents that the complete collection of documents required is available.
Post-condition
The patient’s prenatal care provider delivers by cesarean section after anesthesia. The post-partum discharge planning is notified and assures that there is a suitable environment with appropriate support for post-delivery after-care. Delivery information, i.e. birth weight, APGAR scores, type of delivery, etc is available for pediatrician. The patient's postpartum record is sent to the consultant for incorporation into the patient's record. The patient can incorporate the history and physical into her own personal health record and the newborn’s records into the newborn's personal health record.
Actors/Transaction
There are two actors in this profile, the Content Creator and the Content Consumer. Content is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile. A Document Source or a Portable Media Creator may embody the Content Creator Actor. A Document Consumer, a Document Recipient or a Portable Media Importer may embody the Content Consumer Actor. The sharing or transmission of content or updates from one actor to the other is addressed by the use of appropriate IHE profiles described by section 3.7 Content Bindings with XDS, XDM and XDR found in the Patient Care Coordination Technical Framework
Options
| Actor | Option | Section |
|---|---|---|
| Content Consumer | View Option (See Note 1) Document Import Option (See Note 1) |
PCC TF-1: 2.13.1 PCC TF-1: 2.13.2 |
| Content Creator | Referral Option (See Note 1) Discharge Summary Option (See Note 1) |
PCC TF-1: 2.13.5 PCC TF-1: 2.13.6 |
Note 1: The Actor shall support at least one of these options.
Grouping
Content Bindings with XDS, XDM and XDR
It is expected that the transfers of care will occur in an environment where the physician offices and hospitals have a coordinated infrastructure that serves the information sharing needs of this community of care. Several mechanisms are supported by IHE profiles:
- A registry/repository-based infrastructure is defined by the IHE Cross Enterprise Document Sharing (XDS) and other IHE Integration Profiles such as patient identification (PIX & PDQ), and notification of availability of documents (NAV).
- A media-based infrastructure is defined by the IHE Cross Enterprise Document Media Interchange (XDM) profile.
- A reliable messaging-based infrastructure is defined by the IHE Cross Enterprise Document Reliable Interchange (XDR) profile.
- All of these infrastructures support Security and privacy through the use of the Consistent Time (CT) and Audit Trail and Node Authentication (ATNA) profiles.
For more details on these profiles, see the IHE IT Infrastructure Technical Framework.
Content profiles may impose additional requirements on the transactions used when grouped with actors from other IHE Profiles.
Cross Enterprise Document Sharing, Media Interchange and Reliable Messages
Actors from the ITI XDS, XDM and XDR profiles embody the Content Creator and Content Consumer sharing function of this profile. A Content Creator or Content Consumer must be grouped with appropriate actors from the XDS, XDM or XDR profiles, and the metadata sent in the document sharing or interchange messages has specific relationships to the content of the clinical document described in the content profile.
Notification of Document Availability (NAV)
A Document Source should provide the capability to issue a Send Notification Transaction per the ITI Notification of Document Availability (NAV) Integration Profile in order to notify one or more Document Consumer(s) of the availability of one or more documents for retrieval. One of the Acknowledgement Request options may be used to request from a Document Consumer that an acknowledgement should be returned when it has received and processed the notification. A Document Consumer should provide the capability to receive a Receive Notification Transaction per the NAV Integration Profile in order to be notified by Document Sources of the availability of one or more documents for retrieval. The Send Acknowledgement option may be used to issue a Send Acknowledgement to a Document Source that the notification was received and processed.
Document Digital Signature (DSG)
When a Content Creator Actor needs to digitally sign a document in a submission set, it may support the Digital Signature (DSG) Content Profile as a Document Source. When a Content Consumer Actor needs to verify a Digital Signature, it may retrieve the digital signature document and may perform the verification against the signed document content.
Content Modules
Content modules describe the content of a payload found in an IHE transaction. Content profiles are transaction neutral. They do not have dependencies upon the transaction that they appear in. The Antepartum Record Profile currently consists of four document content modules, with potentially more added in future work. One or more of these document content modules are required for the implementation of APR. For example, a system may choose to implement the Antepartum Summary the first year, and implement the additional content modules of Antepartum History & Physical, Antepartum Laboratory and Antepartum Education is following years.
Antepartum History and Physical
Antepartum Summary
Antepartum Laboratory
Antepartum Education
Process Flow
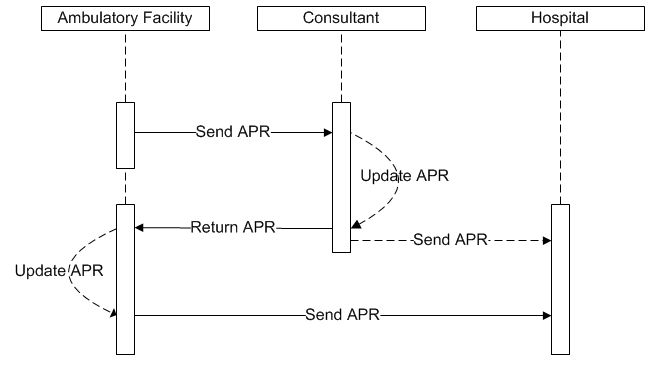
This process flow diagram shows the movement of the antepartum record over the course of care for a pregnancy involving an ambulatory facility (obstetric provider), consultant and hospital (birthing facility). This diagram specifically excludes other infrastructure interactions for simplicity and readability. These infrastructure interactions may be found elsewhere in the framework.
Data from the patient's prenatal care aggregates into her electronic antepartum record by the obstetric provider. The antepartum record is then sent to a consultant who updates the antepartum record, and returns it to the obstetric provider. The electronic antepartum record is then sent to the birthing facility at the appropriate time(s). The consultant may also send the antepartum record directly to the hospital.
Appendix A - Actor Descriptions
Actors are information systems or components of information systems that produce, manage, or act on information associated with operational activities in the enterprise.
- Content Creator
- The Content Creator Actor is responsible for the creation of content and transmission to a Content Consumer.
- Content Consumer
- A Content Consumer Actor is responsible for viewing, import, or other processing of content created by a Content Creator Actor.
- Clinical Data Consumer
- A clinical data consumer makes use of clinical patient data.
- Clinical Data Source
- A Clinical Data Sources maintains patient information about vital signs, problem and allergies, results from diagnostic tests (e.g., Lab, Imaging, or other test results), medications, immunizations or historical or planned visits and procedures.
Appendix B - Transaction Descriptions
Transactions are interactions between actors that transfer the required information through standards-based messages. The PCC Technical Framework does not define any specific transactions, as these are assumed to be carried out through the use of transactions defined in other IHE Profiles.
- Query Existing Data
- Request information about recent patient information, used to obtain vital signs measurements, problems and allergies, diagnostic results, medications, immunizations, or procedures or visits relevant for a patient. The query may request information about some or all of the above topics, or may request information on a specific topic, or one entered for a specific encounter or date range.
Appendix C - How to Prepare an IHE Integration Statement
IHE Integration Statements are documents prepared and published by vendors to describe the conformance of their products with the IHE Technical Framework. They identify the specific IHE capabilities a given product supports in terms of IHE actors and integration profiles described in the technical frameworks of each domain.
Users familiar with these concepts can use Integration Statements to determine what level of integration a vendor asserts a product supports with complementary systems and what clinical and operational benefits such integration might provide. Integration Statements are intended to be used in conjunction with statements of conformance to specific standards (e.g., HL7, IETF, DICOM, W3C, etc.).
IHE provides a process for vendors to test their implementations of IHE actors and integration profiles. The IHE testing process, culminating in a multi-party interactive testing event called the Connectathon, provides vendors with valuable feedback and provides a baseline indication of the conformance of their implementations. The process is not intended to independently evaluate, or ensure, product compliance. In publishing the results of the Connectathon and facilitating access to vendors' IHE Integration Statements, IHE and its sponsoring organizations are in no way attesting to the accuracy or validity of any vendor's IHE Integration Statements or any other claims by vendors regarding their products.
IMPORTANT -- PLEASE NOTE: Vendors have sole responsibility for the accuracy and validity of their IHE Integration Statements. Vendors' Integration Statements are made available through IHE simply for consideration by parties seeking information about the integration capabilities of particular products. IHE and its sponsoring organizations have not evaluated or approved any IHE Integration Statement or any related product, and IHE and its sponsoring organizations shall have no liability or responsibility to any party for any claims or damages, whether direct, indirect, incidental or consequential, including but not limited to business interruption and loss of revenue, arising from any use of, or reliance upon, any IHE Integration Statement.
Structure and Content of an IHE Integration Statement
An IHE Integration Statement for a product shall include:
- The Vendor Name
- The Product Name (as used in the commercial context) to which the IHE Integration Statement applies.
- The Product Version to which the IHE Integration Statement applies.
- A publication date and optionally a revision designation for the IHE Integration Statement.
- The following statement: "This product implements all transactions required in the IHE Technical Framework to support the IHE Integration Profiles, Actors and Options listed below:"
- A list of IHE Integration Profiles supported by the product and, for each Integration Profile, a list of IHE Actors supported. For each integration profile/actor combination, one or more of the options defined in the IHE Technical Framework may also be stated. Profiles, Actors and Options shall use the names defined by the IHE Technical Framework Volume I. (Note: The vendor may also elect to indicate the version number of the Technical Framework referenced for each Integration Profile.)
Note that implementation of the integration profile implies implementation of all required transactions for an actor as well as selected options.
The statement shall also include references and/or internet links to the following information:
- Specific internet address (or universal resource locator [URL]) where the vendor's Integration Statements are posted
- URL where the vendor's standards conformance statements (e.g., HL7, DICOM, etc.) relevant to the IHE transactions implemented by the product are posted.
- URL of the IHE Initiative's web page for general IHE information www.himss.org/ihe.
An IHE Integration Statement is not intended to promote or advertise aspects of a product not directly related to its implementation of IHE capabilities.
Format of an IHE Integration Statement
Each Integration Statement shall follow the format shown below. Vendors may add a cover page and any necessary additional information in accordance with their product documentation policies.
| IHE Integration Statement | Date | 12 Oct 2005 |
| Vendor | Product Name | Version |
| Any Medical Systems Co. | IntegrateRecord | V2.3 |
| This product implements all transactions required in the IHE Technical Framework to support the IHE Integration Profiles, Actors and Options listed below: | ||
| Integration Profiles Implemented | Actors Implemented | Options Implemented |
| Cross-Enterprise Sharing of Medical Summaries | Document Consumer | View Option |
| Audit Trail and Node Authentication | Secure Node | none |
| Patient Identity Cross-referencing | Patient Identifier Cross-reference Consumer | PIX Update Notification |
| Internet address for vendor's IHE information:www.anymedicalsystemsco.com/ihe | ||
| Links to Standards Conformance Statements for the Implementation | ||
| HL7 | www.anymedicalsystemsco.com/hl7 | |
| Links to general information on IHE | ||
| In North America: www.ihe.het | In Europe: www.ihe-europe.org | In Japan: www.jira-net.or.jp/ihe-j |
IHE Integration Statement template
An IHE Integration Statement template (MS Word version) is available here.
The IHE Product Registry
The assumption of an integration statement is that all actors listed are functionally grouped and conform to any profile specifications for such groupings. In case of exceptions the vendor must explicitly describe the functional groupings.
IHE has developed a new Web-based database of Integration Statements. The IHE Product Registry enables developers to create, manage and publish Integration Statements for their commercial and open source healthcare IT systems. It allows users to browse for these systems based on their conformance with specific IHE Actors and Profiles. The system is open for use by developers and users now!
Appendix D - Braden Scale for Predicting Pressure Sore Risk
See File:Braden.pdf
Glossary
The following terms are used in various places within this technical framework, and are defined below. The complete IHE Glossary is available on the IHE Wiki at http://wiki.ihe.net/index.php/IHE_Glossary .
- Actor
- An entity within a use case diagram that can perform an action within a use case diagram. Possible actions are creation or consumption of a message
- Acuity Assessment
Also known as triage category, this is the acuity of the patient assigned during the process of ED triage. A number of evidenced based triage scales exist, including the Emergency Severity Index (ESI), Canadian Triage and Acuity Scale (CTAS), the Australasian Triage Scale (ATS), and the Manchester Triage System. In many emergency departments, patients may simply be classified as emergent, urgent or non-urgent.
- ADT
- Admit, Discharge & Transfer.
- Affinity Domain Policy
- Affinity Domain Policy that clearly defines the appropriate uses of the XDS Affinity Domain. Within this policy is a defined set of acceptable use Privacy Consent Policies that are published and understood.
- ASTM
- Formerly the American Society of Testing and Materials, now ASTM International. An SDO that develops a number of standards across a wide variety of industries, including healthcare.
- ATNA
- Audit Trail and Node Authentication. An IHE ITI profile.
- Care Context
- The participations surrounding the care provision act, and the attributes of that act. Everything in the document header. Data history, links to clinical reasoning.
- Continuity of Care Document(CCD)
- An HL7 Clinical Document Architecture (CDA) implementation alternative to ASTM ADJE2369 for institutions or organizations committed to HL7 standards. This specification was developed as a collaborative effort between ASTM and HL7. More information is available from http://www.hl7.org.
- Continuity of Care Record (CCR)
- A core data set of the most relevant administrative, demographic, and clinical information facts about a patient’s healthcare, covering one or more encounters. The CCR is Designation E2369-05 of the ASTM (American Society for Testing and Materials, International). More information is available from http://www.astm.org.
- Clinical Document Architecture (CDA)
- An HL7 standard for the exchange for clinical documents. It specifies the structure and semantics of clinical documents. More information is available from http://www.hl7.org.
- Content Binding
- A content binding describes how the payload used in an IHE transaction is related to and/or constrained by the data elements contained within the content sent or received in those transactions.
- CRS
- Care Record Summary. An implementation guide that constrains CDA Release 2 for Care Record Summary documents.
- CT
- Consistent Time Integration Profile.
- DICOM
- Digital Imaging and Communication in Medicine
- DSG
- Digital Signatures. An IHE ITI Profile.
- EDIS
- An Emergency Department Information System (EDIS) is an extended EHR system used to manage data in support of Emergency Department patient care and operations. The functions of an EDIS may be provided by a single application or multiple applications.
- eMPI
- Enterprise Master Patient Index.
- EMR
- Electronic Medical Record, an Electronic Health Record system used within an enterprise to deliver care (also called EHR-CR by IHE-XDS).
- Estimated Time of Arrival
- the time the patient being referred can be expected to arrive in the emergency department.
- EUA
- Enterprise User Authentication Integration Profile.
- Expected Actions
- Actions which should occur as the result of a trigger event.
- HIMSS
- Healthcare Information and Management Systems Society.
- HL7
- Health Level Seven
- HIS
- Hospital Information System.
- IHE
- Integrating the Healthcare Enterprise.
- Interaction Diagram
- A diagram that depicts data flow and sequencing of events.
- IT
- Information Technology.
- Logical Observation Identifiers Names and Codes (LOINC®)
- A vocabulary developed by the Regenstrief Institute aimed at standardizing laboratory and clinical codes for use in clinical care, outcomes management, and research. Additional information found at http://www.regenstrief.org/medinformatics/loinc/.
- Mode of Arrival
- The method of transportation used to transport the patient to the Emergency Department.
- MPI
- Master Patient Index.
- MRN
- Medical Record Number.
- NAV
- Notification of Document Availability
- OID
- Object Identifier. (See also 'Globally Unique Identifier').
- Patient Identifier Cross-reference Domain
- Consists of a set of Patient Identifier Domains known and managed by a Patient Identifier Cross-reference Manager Actor. The Patient Identifier Cross-reference Manager Actor is responsible for providing lists of "alias" identifiers from different Patient Identifier Domains.
- Patient Identifier Domain
- A single system or a set of interconnected systems that all share a common identification scheme for patients. Such a scheme includes: (1) a single identifier-issuing authority, (2) an assignment process of an identifier to a patient, (3) a permanent record of issued patient identifiers with associated traits, and (4) a maintenance process over time. The goal of Patient Identification is to reduce errors.
- Portable Document Format.
- PIX
- Patient Identifier Cross Referencing. An IHE ITI Profile.
- PDQ
- Patient Demographics Query. An IHE ITI Profile.
- PHR
- Personal Health Record
- Procedure
- In the context of a "Pre-procedure History and Physical," the "procedure" is a surgery or an invasive examination of a patient that is required by quality review organizations to be preceded by a pre-procedure assessment of procedure risk and anesthesia risk. This assessment is typically referred to as a "Pre-operative" or "Pre-procedure History and Physical."
- Process Flow Diagram
- A graphical illustration of the flow of processes and interactions among the actors involved in a particular example.
- Proposed disposition
- the intended disposition (i.e. admission to ICU, discharge to home, transfer to psychiatric hospital), if known, that the referring provider expects the patient will end up after the emergency department intervention.
- Referral Source
- An individual, group, or agency that determined the patient should seek care at the ED. Referral source may be used to determine appropriate discharge referrals and services, or to provide surveillance data for program and service planning, or to examine referral patterns.
- Role
- The actions of an actor in a use case.
- RSNA
- Radiological Society of North America.
- sig.
- A Latin abbreviation for signetur used to represent the instruction following the medication name.
- Scope
- A brief description of the transaction.
SNOMED-CT® A comprehensive clinical terminology, originally created by the College of American Pathologists (CAP) and, as of April 2007, owned, maintained, and distributed by the International Health Terminology Standards Development Organisation (IHTSDO), a non-for-profit association in Denmark. The CAP continues to support SNOMED CT operations under contract to the IHTSDO and provides SNOMED-related products and services as a licensee of the terminology. More information available from http://www.ihtsdo.org/ or the United States National Library of Medicine at http://www.nlm.nih.gov/research/umls/Snomed/snomed_main.html
- Transport Mode
- the method the patient employs, or is provided to get to the emergency department.
- Trigger Event
- An event such as the reception of a message or completion of a process, which causes another action to occur.
- UID
- Unique Identifier (See also Globally Unique Identifier).
- Universal ID
- Unique identifier over time within the UID type. Each UID must belong to one of specifically enumerated species. Universal ID must follow syntactic rules of its scheme.
- Use Case
- A graphical depiction of the actors and operation of a system.
- XUA
- Cross Enterprise User Authentication
- XDS
- Cross Enterprise Document Sharing
Volume 2
HIMSS and RSNA
Integrating the Healthcare Enterprise
IHE Patient Care Coordination
Antepartum Record (APR)
Technical Framework Supplement
Volume II
Revision 3.0
2008-2009
Public Comment
- (PCC TF-2/Preface)Preface
- (PCC TF-2/Introduction)Introduction
IHE Transactions
This section defines each IHE transaction in detail, specifying the standards used, and the information transferred.
IHE Patient Care Coordination Bindings
This section describes how the payload used in a transaction of an IHE profile is related to and/or constrains the data elements sent or received in those transactions. This section is where any specific dependencies between the content and transaction are defined.
A content integration profile can define multiple bindings. Each binding should identify the transactions and content to which it applies.
The source for all required and optional attributes have been defined in the bindings below. Three tables describe the three main XDS object types: XDSDocumentEntry, XDSSubmissionSet, and XDSFolder. XDSSubmissionSet and XDSDocumentEntry are required. Use of XDSFolder is optional. These concepts are universal to XDS, XDR and XDM.
The columns of the following tables are:
- <XXX> attribute – name of an XDS attribute, followed by any discussion of the binding detail.
- Optional? - Indicates the required status of the XDS attribute, and is one of R, R2, or O (optional). This column is filled with the values specified in the XDS Profile as a convenience.
- Source Type – Will contain one of the following values:
| Source Type | Description |
| SA | Source document Attribute – value is copied directly from source document. The Source/Value column identifies where in the source document this attribute comes from. Specify the location in XPath when possible. |
| SAT | Source document Attribute with Transformation – value is copied from source document and transformed. The Source/Value column identifies where in the source document this attribute comes from. Specify the location in XPath when possible. Extended Discussion column must not be empty and the transform must be defined in the extended discussion |
| FM | Fixed (constant) by Mapping - for all source documents. Source/Value column contains the value to be used in all documents. |
| FAD | Fixed by Affinity Domain – value configured into Affinity Domain, all documents will use this value. |
| CAD | Coded in Affinity Domain – a list of acceptable codes are to be configured into Affinity Domain. The value for this attribute shall be taken from this list. |
| CADT | Coded in Affinity Domain with Transform - a list of acceptable codes are to be configured into Affinity Domain. The value for this attribute shall be taken from this list. |
| n/a | Not Applicable – may be used with an optionality R2 or O attribute to indicate it is not to be used. |
| DS | Document Source – value comes from the Document Source actor. Use Source/Value column or Extended Discussion to give details. |
| O | Other – Extended Discussion must be 'yes' and details given in an Extended Discussion. |
- Source/Value – This column indicates the source or the value used.
The following tables are intended to be summaries of the mapping and transforms. The accompanying sections labeled 'Extended Discussion' are to contain the details as necessary.
Medical Document Binding to XDS, XDM and XDR
This binding defines a transformation that generates metadata for the XDSDocumentEntry element of appropriate transactions from the XDS, XDM and XDR profiles given a medical document and information from other sources. The medical document refers to the document being stored in a repository that will be referenced in the registry. The other sources of information include the configuration of the Document Source actor, the Affinity Domain, the site or facility, local agreements, other documents in the registry/repository, and this Content Profile.
In many cases, the CDA document is created for the purposes of sharing within an affinity domain. In these cases the context of the CDA and the context of the affinity domain are the same, in which case the following mappings shall apply.
In other cases, the CDA document may have been created for internal use, and are subsequentyly being shared. In these cases the context of the CDA document would not neccessarily coincide with that of the affinity domain, and the mappings below would not necessarily apply.
Please note the specifics given in the table below.
XDSDocumentEntry Metadata
| XDSDocumentEntry Attribute | Optional? | Source Type | Source/ Value |
|---|---|---|---|
| availabilityStatus | R | DS | |
| authorInstitution | R2 | SAT |
$inst <=
/ClinicalDocument/author |
| authorPerson | R2 | SAT |
$person <= /ClinicalDocument/author
|
| authorRole | R2 | SAT | This metadata element should be based on a mapping of the participation function defined in the CDA document to the set of author roles configured for the affinity domain. If the context of the CDA coincides with that of the affinity domain, then the following x-path may be appropriate: /ClincicalDocument/author/ participationFunction |
| authorSpecialty | R2 | SAT | This metadata element should be based on a mapping of the code associated with the assignedAuthor to detailed defined classification system for healthcare providers such configured in the affinitity domain. Possible classifications include those found in SNOMED-CT, or the HIPAA Healthcare Provider Taxonomy. If the context of the CDA coincides with that of the affinity domain, then the following x-path may be appropriate: /ClinicalDocument/author/ assignedAuthor/code |
| classCode | R | CADT | Derived from a mapping of /ClinicalDocument/code/@code to an Affinity Domain specified coded value to use and coding system. Affinity Domains are encouraged to use the appropriate value for Type of Service, based on the LOINC Type of Service (see Page 53 of the LOINC User's Manual). Must be consistent with /ClinicalDocument/code/@code |
| classCodeDisplayName | R | CADT | DisplayName of the classCode derived. Derived from a mapping of /ClinicalDocument/code/@code to the appropriate Display Name based on the Type of Service. Must be Consitent with /ClinicalDocument/code/@code |
| confidentialityCode | R | CADT | Derived from a mapping of /ClinicalDocument/confidentialityCode/@code to an Affinity Domain specified coded value and coding system. When using the BPPC profile, the confidentialyCode may also be obtained from the <authorization> element.
|
| comments | O | DS | |
| creationTime | R | SAT | /ClinicalDocument/effectiveTime
|
| entryUUID | R | DS | |
| eventCodeList | O | CADT | These values express a collection of keywords that may be relevant to the consumer of the documents in the registry. They may come from anywhere in the CDA document, according to its purpose. |
| eventCodeDisplayNameList | R (if event Code is valued) |
CADT | These are the display names for the collection of keywords described above. |
| formatCode | R | FM | The format code for each PCC Document content profile is provided within the document specifications. |
| healthcareFacilityTypeCode | R | CAD | A fixed value assigned to the Document Source and configured form a set of Affinity Domain defined values. Must be concistent with /clinicalDocument/code |
| healthcareFacility TypeCodeDisplay Name |
R | CAD | Must be concistent with /clinicalDocument/code |
| intendedRecipient (for XDR, XDM) | O | SAT |
$person <=
/ClinicalDocument/intendedRecipient
"^^^^^&", |
| languageCode | R | SA | /ClinicalDocument/languageCode |
| legalAuthenticator | O | SAT | $person <= /ClinicalDocument/ legalAuthenticator
|
| mimeType | R | FM | text/xml |
| parentDocumentRelationship | R (when applicable) |
DS | Local document versions need not always be published, and so no exact mapping can be determined from the content of the CDA document. The parentDocumentRelationship may be determined in some configurations from the relatedDocument element present in the CDA dsocument. If the context of the CDA coincides with that of the affinity domain, then the following x-path may be appropriate: /ClinicalDocument/relatedDocument/@typeCode |
| parentDocumentId | R (when parent Document Relationship is present) |
DS | Local document versions need not always be published, and so no exact mapping can be determined from the content of the CDA document. The parentDocumentId may be determined in some configurations from the relatedDocument element present in the CDA dsocument. If the context of the CDA coincides with that of the affinity domain, then the following x-path may be appropriate: $docID <= /ClinicalDocument/ relatedDocument/parentDocument/id
|
| patientId | R | DS | The XDS Affinity Domain patient ID can be mapped from the patientRole/id element using transactions from the ITI PIX or PDQ profiles. See sourcePatientId below. If the context of the CDA coincides with that of the affinity domain, then the following x-path may be appropriate:
|
| practiceSettingCode | R | CAD | This elements should be based on a coarse classification system for the class of specialty practice. Recommend the use of the classification system for Practice Setting, such as that described by the Subject Matter Domain in LOINC. |
| practiceSettingCodeDisplayName | R | CAD | This element shall contain the display names associated with the codes described above. |
| serviceStartTime | R2 | SAT | /ClinicalDocument/documentationOf/ serviceEvent/effectiveTime/low/ @value
|
| serviceStopTime | R2 | SAT | /ClinicalDocument/documentationOf/ serviceEvent/effectiveTime/high/ @value
|
| sourcePatientId | R | SAT | $patID <= /ClinicalDocument/recordTarget/ patientRole/id
|
| sourcePatientInfo | R | SAT | /ClinicalDocument/recordTarget/ patientRole
|
| title | O | SA | /ClinicalDocument/title |
| typeCode | R | CADT | /ClinicalDocument/code/@code
|
| typeCodeDisplay Name |
R | CADT | /ClinicalDocument/code/@displayName |
| uniqueId | R | SAT | $docID <= /ClinicalDocument/id
|
XDSSubmissionSet Metadata
The submission set metadata is as defined for XDS, and is not necessarily affected by the content of the clinical document. Metadata values in an XDSSubmissionSet with names identical to those in the XDSDocumentEntry may be inherited from XDSDocumentEntry metadata, but this is left to affinity domain policy and/or application configuration.
Use of XDS Submission Set
This content format uses the XDS Submission Set to create a package of information to send from one provider to another. All documents referenced by the Medical Summary in this Package must be in the submission set.
Use of XDS Folders
No specific requirements identified.
Configuration
IHE Content Profiles using this binding require that Content Creators and Content Consumers be configurable with institution and other specific attributes or parameters. Implementers should be aware of these requirements to make such attributes easily configurable. There shall be a mechanism for the publishing and distribution of style sheets used to view clinical documents.
Extensions from other Domains
Scanned Documents (XDS-SD)
XDS-SD is a CDA R2 document and thus conforms to the XDS Metadata requirements in the PCC-TF, volume 2, Section 5 unless otherwise specified below.
XDSDocumentEntry
XDS-SD leverages the XDS DocumentEntry Metadata requirements in the PCC-TF, volume 2, Section 5.1.1.1.1 and in PCC_TF-2/Bindings unless otherwise specified below
XDSDocumentEntry.formatCode
The XDSDocumentEntry.formatCode shall be urn:ihe:iti:xds-sd:pdf:2008 when the document is scanned pdf and urn:ihe:iti:xds-sd:text:2008 when the document is scanned text. The formatCode codeSystem shall be 1.3.6.1.4.1.19376.1.2.3.
XDSDocumentEntry.uniqueId
This value shall be the ClinicalDocument/id in the HL7 CDA R2 header. The root attribute is required, and the extension attribute is optional. In accordance with the XDS.a profile, total length is limited to 128 characters; for XDS.b the limit is 256 characters. Additionally see PCC-TF, volume 2, Section 5.1.1.1.1 or PCC_TF-2/Bindings for further content specification.
Relating instances of XDS-SD documents
In general, most instances of XDS-SD will not have parent documents. It is possible, however, in some specific use cases that instances of XDS-SD documents are related. For example, for a particular document it may be the case that both the PDF scanned content and somewhat equivalent plaintext need to be wrapped and submitted. Each document would correspond to separate XDSDocumentEntries linked via an XFRM Association that indicates one document is a transform of the other. These can be submitted in a single submission set, or in separate ones. Other specific examples may exist and this profile does not preclude the notion of a parent document for these cases.
XDSSubmissionSet
No additional constraints. Particular to this profile, a legitimate use of submission sets would be to maintain a logical grouping of multiple XDS-SD documents. We encourage such usage. For more information, see PCC-TF-2 Section 5.1.1.1.2 or PCC_TF-2/Bindings.
XDSFolder
No additional requirements. For more information, see PCC-TF-2 Section 5.1.1.1.3 or PCC_TF-2/Bindings.
Basic Patient Privacy Consents (BPPC)
Laboratory Reports (XD-LAB)
XD-Lab is a CDA R2 document and thus conforms to the XDS Metadata requirements in the PCC-TF, volume 2, Section 5 unless otherwise specified below.
XDSDocumentEntry
XD-Lab leverages the XDS DocumentEntry Metadata requirements in the PCC-TF, volume 2, Section 5.1.1.1.1 and in PCC_TF-2/Bindings unless otherwise specified below
XDSDocumentEntry.eventCodeList
XD-Lab documents further constrain the the XDSDocumentEntry.eventCodeList to the following.
| Attribute | Optional? | Source Type | Source/ Value |
|---|---|---|---|
| eventCodeList | R2 | SAT | ClinicalDocument / component / structuredBody / component / section / entry / act / entryRelationship / organizer (templateId="1.3.6.1.4.1.19376.1.3.1.1")/ component / observation(templateId="1.3.6.1.4.1.19376.1.3.1.1.1")/code
AND ClinicalDocument / component / structuredBody / component / section / entry / act / subject / code If the document has Reportable Condition, then this code shall be among those listed in the eventCodeList. Additionally, if the document contains information about a Non-Human Subject, then the code that indicates what this subject is shall be among those listed in the eventCodeList. Thus, this attribute has been enhanced from the XDS profile from O to R2. |
XDSDocumentEntry.formatCode
The XDSDocumentEntry.formatCode shall be urn:ihe:lab:xd-lab:2008 The formatCode codeSystem shall be 1.3.6.1.4.1.19376.1.2.3.
XDSSubmissionSet
No additional constraints. For more information, see PCC-TF-2 Section 5.1.1.1.2 or PCC_TF-2/Bindings.
XDSFolder
No additional requirements. For more information, see PCC-TF-2 Section 5.1.1.1.3 or PCC_TF-2/Bindings.
Namespaces and Vocabularies
This section lists the namespaces and identifiers defined or referenced by the IHE PCC Technical Framework, and the vocabularies defined or referenced herein.
The following vocabularies are referenced in this document. An extensive list of registered vocabularies can be found at http://www.hl7.org/oid/.
| codeSystem | codeSystemName | Description |
| 1.3.6.1.4.1.19376.1.5.3.1 | IHE PCC Template Identifiers | This is the root OID for all IHE PCC Templates. A list of PCC templates can be found below in CDA Release 2.0 Content Modules. |
| 1.3.6.1.4.1.19376.1.5.3.2 | IHEActCode | See IHEActCode Vocabulary below |
| 1.3.6.1.4.1.19376.1.5.3.3 | IHE PCC RoleCode | See IHERoleCode Vocabulary below |
| 1.3.6.1.4.1.19376.1.5.3.4 | Namespace OID used for IHE Extensions to CDA Release 2.0 | |
| 2.16.840.1.113883.10.20.1 | CCD Root OID | Root OID used for by ASTM/HL7 Continuity of Care Document |
| 2.16.840.1.113883.5.112 | RouteOfAdministration | See the HL7 RouteOfAdministration Vocabulary |
| 2.16.840.1.113883.5.1063 | SeverityObservation | See the HL7 SeverityObservation Vocabulary |
| 2.16.840.1.113883.5.7 | ActPriority | See the HL7 ActPriority Vocabulary |
| 2.16.840.1.113883.6.1 | LOINC | Logical Observation Identifier Names and Codes |
| 2.16.840.1.113883.6.96 | SNOMED-CT | SNOMED Controlled Terminology |
| 2.16.840.1.113883.6.103 | ICD-9CM (diagnosis codes) | International Classification of Diseases, Clinical Modifiers, Version 9 |
| 2.16.840.1.113883.6.104 | ICD-9CM (procedure codes) | International Classification of Diseases, Clinical Modifiers, Version 9 |
| 2.16.840.1.113883.6.26 | MEDCIN | A classification system from MEDICOMP Systems. |
| 2.16.840.1.113883.6.88 | RxNorm | RxNorm |
| 2.16.840.1.113883.6.63 | FDDC | First DataBank Drug Codes |
| 2.16.840.1.113883.6.12 | C4 | Current Procedure Terminology 4 (CPT-4) codes. |
| 2.16.840.1.113883.6.257 | Minimum Data Set for Long Term Care | The root OID for Minimum Data Set Answer Lists |
| 1.2.840.10008.2.16.4 | DCM | DICOM Controlled Terminology; PS 3.16 Content Mapping Resource, Annex D |
| 2.16.840.1.113883.6.24 | MDC | ISO/IEEE 11073 Medical Device Nomenclature |
| 2.16.840.1.113883.3.26.1.5 | NDF-RT | National Drug File Reference Terminology (NCI version) |
| 2.16.840.1.113883.11.19465 | nuccProviderCodes | National Uniform Codes Council Healthcare Provider Terminology |
| 2.16.840.1.113883.6.255.1336 | X12DE1336 | Insurance Type Code (ASC X12 Data Element 1336) |
| 2.16.840.1.113883.6.256 | RadLex | RadLex (Radiological Society of North America) |
The IHE FormatCode vocabulary is now managed in an Implementation Guide published using FHIR.
- formal canonical URI https://profiles.ihe.net/fhir/ihe.formatcode.fhir/ValueSet-formatcode.html
- formal publication URL https://profiles.ihe.net/fhir/ihe.formatcode.fhir/
- FormatCode gitHub repository for source of the Implementation Guide can be used to register issues, or create pull requests for modifications. Formal governance is managed by ITI Technical Committee.
This FormatCode vocabulary represents:
- Code System 1.3.6.1.4.1.19376.1.2.3
- Value Set 1.3.6.1.4.1.19376.1.2.7.1
IHEActCode Vocabulary
| CCD | ASTM/HL7 Continuity of Care Document | |
| CCR | ASTM CCR Implementation Guide |
The IHEActCode vocabulary is a small vocabulary of clinical acts that are not presently supported by the HL7 ActCode vocabulary. The root namespace (OID) for this vocabulary is 1.3.6.1.4.1.19376.1.5.3.2. These vocabulary terms are based on the vocabulary and concepts used in the CCR and CCD standards listed above.
| Code | Description |
| COMMENT | This is the act of commenting on another act. |
| PINSTRUCT | This is the act of providing instructions to a patient regarding the use of medication. |
| FINSTRUCT | This is the act of providing instructions to the supplier regarding the fulfillment of the medication order. |
| IMMUNIZ | The act of immunization of a patient using a particular substance or class of substances identified using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| DRUG | The act of treating a patient with a particular substance or class of substances identified using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| INTOL | An observation that a patient is somehow intollerant of (e.g., allergic to) a particular substance or class of substances using a specified vocabulary. Use of this vocabulary term requires the use of either the SUBSTANCE or SUBSTCLASS qualifier described below, along with an identified substance or class of substances. |
| SUBSTANCE | A qualifier that identifies the substance used to treat a patient in an immunization or drug treatment act. The substance is expected to be identified using a vocabulary such as RxNORM, SNOMED CT or other similar vocabulary and should be specific enough to identify the ingredients of the substance used. |
| SUBSTCLASS | A qualifier that identifies the class of substance used to treat a patient in an immunization or drug treatment act. The class of substances is expected to be identified using a vocabulary such as NDF-RT, SNOMED CT or other similar vocabulary, and should be broad enough to classify substances by mechanism of action (e.g., Beta Blocker), intended effect (Dieuretic, antibiotic) or ... |
| For Public Comment | What else needs to appear above for SUBSTCLASS? |
IHERoleCode Vocabulary
The IHERoleCode vocabulary is a small vocabulary of role codes that are not presently supported by the HL7 Role Code vocabulary. The root namespace (OID) for this vocabulary is 1.3.6.1.4.1.19376.1.5.3.3.
| Code | Description |
| EMPLOYER | The employer of a person. |
| SCHOOL | The school in which a person is enrolled. |
| AFFILIATED | An organization with which a person is affiliated (e.g., a volunteer organization). |
| PHARMACY | The pharmacy a person uses. |
PCC Content Modules
This chapter contains the various content modules and value sets that are used with IHE Patient Care Coordintation profiles and transactions.
Conventions
Various tables used in this section will further constrain the content. Within this volume, the follow conventions are used.
- R
- A "Required" data element is one that shall always be provided. If there is information available, the data element must be present. If there is no information available, or it cannot be transmitted, the data element must contain a value indicating the reason for omission of the data. (See PCC TF-2: 5.3.4.2 for a list of appropriate statements).
- R2
- A "Required if data present" data element is one that shall be provided when a value exists. If the information cannot be transmitted, the data element shall contain a value indicating the reason for omission of the data. If no such information is available to the creator or if such information is not available in a well identified manner (e.g. buried in a free form narrative that contains additional information relevant to other sections) or if the creator requires that information be absent, the R2 section shall be entirely absent. (See section PCC TF-2: 5.3.4.2 for a list of appropriate statements).
- O
- An optional data element is one that may be provided, irrespective of whether the information is available or not. If the implementation elects to support this optional section, then its support shall meet the requirement set forth for the "Required if data present" or R2.
- C
- A conditional data element is one that is required, required if known or optional depending upon other conditions. These will have further notes explaining when the data element is required, et cetera.
| Note: | The definitions of R, R2, and O differ slightly from other IHE profiles. This is due in part to the fact that local regulations and policies may in fact prohibit the transmission of certain information, and that a human decision to transmit the information may be required in many cases. |
Folder Content Modules
This section contains modules that describe the content requirements of Folders used with XDS, XDM or XDR. When workflows are completed normally, the folders will contain documents with the optionality specified in the tables shown below. Under certain circumstances, the folders will not meet the optionality requirements described below, for example, when the patient leaves before treatment is completed.
- (X-AntepartumRecords)APR Folder
HL7 Version 3.0 Content Modules
This section contains content modules based upon the HL7 CDA Release 2.0 Standard, and related standards and/or implementation guides.
CDA Document Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.1.1)Medical Documents Specification
- (1.3.6.1.4.1.19376.1.5.3.1.1.2)Medical Summary Specification
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.1.4)History and Physical
- (1.3.6.1.4.1.19376.1.5.3.1.1.11.2)Antepartum Summary
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.1.1)Antepartum History and Physical
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.1.2)Antepartum Laboratory
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.1.3)Antepartum Education
CDA Header Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.2.4)PCC TF 2:6.3.2.4 Patient Contacts
- (1.3.6.1.4.1.19376.1.5.3.1.2.4.1)PCC CDA Supplement 2:6.3.2.5 Spouse
- (1.3.6.1.4.1.19376.1.5.3.1.2.4.2)PCC CDA Supplement 2:6.3.2.6 Natural Father of Fetus
- (1.3.6.1.4.1.19376.1.5.3.1.2.5)PCC CDA Supplement 2:6.3.2.7 Authorization
CDA Section Content Modules
This list defines the sections that may appear in a medical document. It is intended to be a comprehensive list of all document sections that are used by any content profile defined in the Patient Care Coordination Technical Framework. All sections shall have a narrative component that may be freely formatted into normal text, lists, tables, or other appropriate human-readable presentations. Additional subsections or entry content modules may be required.
Reasons for Care
The sections described below describe various reasons why healthcare is being provided to the patient.
- (1.3.6.1.4.1.19376.1.5.3.1.1.13.2.1)PCC TF 2:6.3.3.1.3 Chief Complaint
Other Condition Histories
The sections defined below provide historical information about the patient's conditions.
- (1.3.6.1.4.1.19376.1.5.3.1.3.4)PCC TF 2:6.3.3.2.1 History of Present Illness
- (1.3.6.1.4.1.19376.1.5.3.1.3.6)PCC TF 2:6.3.3.2.3 Active Problems
- (1.3.6.1.4.1.19376.1.5.3.1.3.8)PCC TF 2:6.3.3.2.5 History of Past Illness
- (1.3.6.1.4.1.19376.1.5.3.1.3.13)PCC TF 2:6.3.3.2.11 Allergies and Other Adverse Reactions
- (1.3.6.1.4.1.19376.1.5.3.1.3.14)PCC TF 2:6.3.3.2.12 Family Medical History
- (1.3.6.1.4.1.19376.1.5.3.1.3.15)PCC TF 2:6.3.3.2.13 Coded Family Medical History
- (1.3.6.1.4.1.19376.1.5.3.1.3.16)PCC TF 2:6.3.3.2.14 Social History
- (1.3.6.1.4.1.19376.1.5.3.1.3.18)PCC TF 2:6.3.3.2.16 Review of Systems
- (1.3.6.1.4.1.19376.1.5.3.1.1.5.3.4)PCC TF 2:6.3.3.2.18 Pregnancy History
- (1.3.6.1.4.1.19376.1.5.3.1.1.11.2.2.1)PCC CDA Supplement 2:6.3.3.2.28 Estimated Delivery Dates
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.2.1.1)PCC CDA Supplement 2:6.3.3.2.35 History of Infection
Medications
This section contains section content modules that describe activities surrounding the use of medication.
- (1.3.6.1.4.1.19376.1.5.3.1.3.19)PCC TF 2:6.3.3.3.1 Medications
- (1.3.6.1.4.1.19376.1.5.3.1.3.23)PCC TF 2:6.3.3.3.5 Immunizations
Physical Exams
- (1.3.6.1.4.1.19376.1.5.3.1.3.24)PCC TF 2:6.3.3.4.1 Physical Exam
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.15)PCC TF 2:6.3.3.4.2 Physical Exam (with subsections)
- (1.3.6.1.4.1.19376.1.5.3.1.3.25)PCC TF 2:6.3.3.4.4 Vital Signs
- (1.3.6.1.4.1.19376.1.5.3.1.1.5.3.2)PCC TF 2:6.3.3.4.5 Coded Vital Signs
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.16)PCC TF 2:6.3.3.4.6 General Appearance
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.17)PCC TF 2:6.3.3.4.8 Integumentary System
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.18)PCC TF 2:6.3.3.4.9 Head
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.19)PCC TF 2:6.3.3.4.10 Eyes
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.20)PCC TF 2:6.3.3.4.11 Ears, Nose, Mouth and Throat
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.21)PCC TF 2:6.3.3.4.12 Ears
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.22)PCC TF 2:6.3.3.4.13 Nose
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.23)PCC TF 2:6.3.3.4.14 Mouth, Throat, and Teeth
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.24)PCC TF 2:6.3.3.4.15 Neck
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.25)PCC TF 2:6.3.3.4.16 Endocrine System
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.26)PCC TF 2:6.3.3.4.17 Thorax and Lungs
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.27)PCC TF 2:6.3.3.4.18 Chest Wall
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.28)PCC TF 2:6.3.3.4.19 Breasts
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.29)PCC TF 2:6.3.3.4.20 Heart
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.30)PCC TF 2:6.3.3.4.21 Respiratory System
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.31)PCC TF 2:6.3.3.4.22 Abdomen
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.32)PCC TF 2:6.3.3.4.23 Lymphatic System
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.33)PCC TF 2:6.3.3.4.24 Vessels
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.34)PCC TF 2:6.3.3.4.25 Musculoskeletal System X
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.35)PCC TF 2:6.3.3.4.26 Neurologic System
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.36)PCC TF 2:6.3.3.4.27 Genitalia
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.37)PCC TF 2:6.3.3.4.28 Rectum
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.2.1)PCC TF 2:6.3.3.4.29 Extremeties
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.15.1)PCC TF 2:6.3.3.4.30 Coded Physical Exam
- (1.3.6.1.4.1.19376.1.5.3.1.1.21.2.10)PCC TF 2:6.3.3.4.31 Pelvis
Relevant Studies
- (1.3.6.1.4.1.19376.1.5.3.1.3.27)PCC TF 2:6.3.3.5.1 Results
- (1.3.6.1.4.1.19376.1.5.3.1.3.28)PCC TF 2:6.3.3.5.2 Coded Results
Plans of Care
This section provides content modules for sections that describe the plan of care intended for the patient.
- (1.3.6.1.4.1.19376.1.5.3.1.3.31)PCC TF 2:6.3.3.6.1 Care Plan
- (1.3.6.1.4.1.19376.1.5.3.1.1.13.2.5)PCC TF 2:6.3.3.6.2 Assessment and Plan
- (1.3.6.1.4.1.19376.1.5.3.1.3.34)PCC TF 2:6.3.3.6.5 Advance Directives
- (1.3.6.1.4.1.19376.1.5.3.1.3.35)PCC TF 2:6.3.3.6.6 Coded Advance Directives
Procedures Performed
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.38)PCC CDA Supplement 2:6.3.3.8.1 Patient Education and Consents
- (1.3.6.1.4.1.19376.1.5.3.1.1.9.39)PCC CDA Supplement 2:6.3.3.8.2 Coded Patient Education and Consents
Impressions
- (1.3.6.1.4.1.19376.1.5.3.1.1.11.2.2.2)PCC CDA Supplement 2:6.3.3.9.3 Visit Summary
- (1.3.6.1.4.1.19376.1.5.3.1.1.13.2.4)PCC CDA Supplement 2:6.3.3.9.7 Assessments
CDA and HL7 Version 3 Entry Content Modules
- (1.3.6.1.4.1.19376.1.5.3.1.4.1)PCC TF 2:6.3.4.3 Severity
- (1.3.6.1.4.1.19376.1.5.3.1.4.1.1)PCC TF 2:6.3.4.4 Problem Status Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.1.2)PCC TF 2:6.3.4.5 Health Status
- (1.3.6.1.4.1.19376.1.5.3.1.4.2)PCC TF 2:6.3.4.6 Comments
- (1.3.6.1.4.1.19376.1.5.3.1.4.3)PCC TF 2:6.3.4.7 Patient Medication Instructions
- (1.3.6.1.4.1.19376.1.5.3.1.4.3.1)PCC TF 2:6.3.4.8 Medication Fulfillment Instructions
- (1.3.6.1.4.1.19376.1.5.3.1.4.4)PCC TF 2:6.3.4.9 External References
- (1.3.6.1.4.1.19376.1.5.3.1.4.4.1)PCC TF 2:6.3.4.10 Internal References
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.1)PCC TF 2:6.3.4.11 Concern Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.2)PCC TF 2:6.3.4.12 Problem Concern Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.5.3)PCC TF 2:6.3.4.13 Allergy and Intolerance Concern
- (1.3.6.1.4.1.19376.1.5.3.1.4.5)PCC TF 2:6.3.4.14 Problem Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.6)PCC TF 2:6.3.4.15 Allergies and Intolerances
- (1.3.6.1.4.1.19376.1.5.3.1.4.7)PCC TF 2:6.3.4.16 Medications
- (1.3.6.1.4.1.19376.1.5.3.1.4.12)PCC TF 2:6.3.4.17 Immunizations
- (1.3.6.1.4.1.19376.1.5.3.1.4.7.3)PCC TF 2:6.3.4.18 Supply Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.7.2)PCC TF 2:6.3.4.19 Product Entry
- (1.3.6.1.4.1.19376.1.5.3.1.4.13)PCC TF 2:6.3.4.20 Simple Observations
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.1)PCC TF 2:6.3.4.21 Vital Signs Organizer
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.2)PCC TF 2:6.3.4.22 Vital Signs Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.15)PCC TF 2:6.3.4.23 Family History Organizer
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.4)PCC TF 2:6.3.4.24 Social History Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.3)PCC CDA Supplement 2:6.3.4.25 Family History Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.5)PCC CDA Supplement 2:6.3.4.26 Pregnancy Observation
- (1.3.6.1.4.1.19376.1.5.3.1.1.11.2.3.1)PCC CDA Supplement 2:6.3.4.27 EDD Observation
- (1.3.6.1.4.1.19376.1.5.3.1.1.11.2.3.2)PCC CDA Supplement 2:6.3.4.28 Antepartum Visit Summary Battery
- (1.3.6.1.4.1.19376.1.5.3.1.4.13.7)PCC CDA Supplement 2:6.3.4.29 Advance Directive Observation
- (1.3.6.1.4.1.19376.1.5.3.1.4.14)PCC CDA Supplement 2:6.3.4.31 Encounters
- (1.3.6.1.4.1.19376.1.5.3.1.4.19)PCC CDA Supplement 2:6.3.4.33 Procedure Entry
- (1.3.6.1.4.1.19376.1.5.3.1.1.12.3.1)PCC CDA Supplement 2:6.3.4.38 Pain Score Observation
HL7 Version 2 Content Modules
This section contains content modules based upon the HL7 Version 2 Standard, and related standards and/or implementation guides.
PCC Value Sets
This section contains value sets used by Content Modules.
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.1)APR History of Past Illness Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.2)APR Pregnancy History Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.3)APR Social History Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.4)APR Family History and Genetic Screening Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.5)APR Menstrual History Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.6)APR History of Infection Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.7)APR Laboratory LOINC Code Value Set
- (1.3.6.1.4.1.19376.1.5.3.1.1.16.5.8)APR Education Value Set
Appendix A - Examples Using PCC Content Profiles
Example documents conforming to each profile can be found on the IHE wiki at the following URLs.
| Profile and Content | URL |
|---|---|
| XDS-MS | |
| Referral Summary | XDSMS Example1 |
| Discharge Summary | XDSMS Example1 |
| XPHR | |
| XPHR Content | XPHR Example1 |
| XPHR Update | XPHR Example2 |
| (EDR) ED Referral | EDR Example |
| (APS) Antepartum Summary | APS Example |
| (EDES) | |
| Triage Note | EDES Example1 |
| ED Nursing Note | EDES Example2 |
| Composite Triage and Nursing Note | EDES Example3 |
| ED Physician Note | EDES Example4 |
| (FSA) Functional Status Section | FSA Example |
Appendix B - Validating CDA Documents using the Framework
Many of the constraints specified by the content modules defined in the PCC Technical Framework can be validated automatically by software. Automated validation is a very desirable capability, as it makes it easier for implementers to test the correctness of their implementations. With regard to validation of the content module, the PCC Technical Framework narrative is the authoritative specification, not any automated software tool. Having said that, it is still very easy to create a validation framework for the IHE PCC Technical Framework using a XML validation tool such as Schematron. Since each content module has a name (the template identifier), any XML instance that reports itself to be of that "class" can be validated by creating assertions that must be true for each constraint indicated for the content module. In the XML representation, the <templateId> element is a child of the element that is claiming conformance to the template named. Thus the general pattern of a Schematron that validates a specific template is shown below:
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReferralSummary'>
<rule context='*[cda:templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.1.3]"'>
<!-- one or more assertions made by the content module -->
</rule>
</pattern>
</schema>
Validating Documents
For document content modules, the pattern can be extended to support common document content module constraints as shown below:
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReferralSummary'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.1.3]"'>
<!-- Verify that the template id is used on the appropriate type of object -->
<assert test='../ClinicalDocument'>
Error: The referral content module can only be used on Clinical Documents.
</assert>
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.1.2"]'>
Error: The parent template identifier for medical summary is not present.
</assert>
<!-- Verify the document type code -->
<assert test='code[@code = "34133-9"]'>
Error: The document type code of a referral summary must be
34133-9 SUMMARIZATION OF EPISODE NOTE.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The document type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
<!-- Verify that all required data elements are present -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: A referral summary must contain a reason for referral.
</assert>
<!-- Alert on any missing required if known elements -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.8"]'>
Warning: A referral summary should contain a list of history of past illnesses.
</assert>
<!-- Note any missing optional elements -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.3.18"]'>
Note: This referral summary does not contain the pertinent review of systems.
</assert>
</rule>
</pattern>
</schema>
Validating Sections
The same pattern can be also applied to sections with just a few minor alterations.
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<pattern name='ReasonForReferralUncoded'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<!-- Verify that the template id is used on the appropriate type of object -->
<assert test='section'>
Error: The coded reason for referral module can only be used on a section.
</assert>
<assert test='false'>
Manual: Manually verify that this section contains narrative providing the
reason for referral.
</assert>
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral
module is not present.
</assert>
<!-- Verify the section type code -->
<assert test='code[@code = "42349-1"]'>
Error: The section type code of the reason for referral section must be 42349-1
REASON FOR REFERRAL.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The section type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
</pattern>
<pattern name='ReasonForReferralCoded'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.2"]'>
<!-- The parent template will have already verified the type of object -->
<!-- Verify that the parent templateId is also present. -->
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral
module is not present.
</assert>
<!-- Don't bother with the section type code, as the parent template caught it -->
<!-- Verify that all required data elements are present -->
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.4.13"]'>
Error: A coded reason for referral section must contain an simple observation.
</assert>
<!-- Alert on any missing required if known elements -->
<!-- Note any missing optional elements -->
</rule>
</pattern>
</schema>
A similar pattern can also be followed for Entry and Header content modules, and these are left as an exercise for the reader.
Phases of Validation and Types of Errors
Note that each message in the Schematrons shown above start with a simple text string that indicates whether the message indicates one of the following conditions:
- An error, e.g., the failure to transmit a required element,
- A warning, e.g., the failure to transmit a required if known element,
- A note, e.g., the failure to transmit an optional element.
- A manual test, e.g., a reminder to manually verify some piece of content.
Schematron supports the capability to group sets of rules into phases by the pattern name, and to specify which phases of validation should be run during processing. To take advantage of this capability, one simply breaks each <pattern> element above up into separate patterns depending upon whether the assertion indicates an error, warning, note or manual test, and then associate each pattern with a different phase. This is shown in the figure below.
<schema xmlns="http://www.ascc.net/xml/schematron" xmlns:cda="urn:hl7-org:v3">
<ns prefix="cda" uri="urn:hl7-org:v3" />
<phase id="errors">
<active pattern="ReasonForReferralUncoded_Errors"/>
<active pattern="ReasonForReferralCoded_Errors"/>
</phase>
<phase id="manual">
<active pattern="ReasonForReferralUncoded_Manual"/>
</phase>
<pattern name='ReasonForReferralUncoded_Errors'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<assert test='section'>
Error: The coded reason for referral module can only be used on a section.
</assert>
<assert test='code[@code = "42349-1"]'>
Error: The section type code of the reason for referral section must be 42349-1
REASON FOR REFERRAL.
</assert>
<assert test='code[@codeSystem = "2.16.840.1.113883.6.1"]'>
Error: The section type code must come from the LOINC code
system (2.16.840.1.113883.6.1).
</assert>
</rule>
</pattern>
<pattern name='ReasonForReferralUncoded_Manual'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
<assert test='false'>
Manual: Manually verify that this section contains narrative providing the
reason for referral.
</assert>
</pattern>
<pattern name='ReasonForReferralCoded_Errors'>
<rule context='*[templateId/@root="1.3.6.1.4.1.19376.1.5.3.1.3.2"]'>
<assert test='templateId[@root="1.3.6.1.4.1.19376.1.5.3.1.3.1"]'>
Error: The parent template identifier for the reason for referral not present.
</assert>
<assert test='.//templateId[@root = "1.3.6.1.4.1.19376.1.5.3.1.4.13"]'>
Error: A coded reason for referral section must contain an simple observation.
</assert>
</rule>
</pattern>
</schema>
Using these simple "templates" for template validation one can simply create a collection of Schematron patterns that can be used to validate the content modules in the PCC Technical Framework. Such Schematrons are expected to be made available as part of the MESA test tools that are provided to IHE Connectathon participants, and which will also be made available to the general public after connectathon.
Appendix C - Extensions to CDA Release 2.0
This section describes extensions to CDA Release 2.0 that are used by the IHE Patient Care Coordination Technical Framework.
IHE PCC Extensions
All Extensions to CDA Release 2.0 created by the IHE PCC Technical Committee are in the namespace urn:ihe:pcc:hl7v3.
The approach used to create extension elements created for the PCC Technical Framework is the same as was used for the HL7 Care Record Summary (see Appendix E) and the ASTM/HL7 Continuity of Care Document (see secion 7.2).
replacementOf
The <replacementOf> extension element is applied to a section appearing in a PHR Update Document to indicate that that section's content should replace that of a previously existing section. The identifier of the previously existing section is given so that the PHR Manager receiving the Update content will know which section to replace. The model for this extension is shown below.
Use of this extension is shown below. The <replacementOf> element appears after all other elements within the <section> element. The <id> element appearing in the <externalDocumentSection> element shall provide the identifier of the section being replaced in the parent document.
<section>
<id root=' ' extension=' '/>
|
Extensions Defined Elsewhere used by IHE PCC
Entity Identifiers
There is often a need to record an identifer for an entity so that it can be subsequently referenced. This extension provides a mechnism to store that identifier. The element appears after any <realm>, <typeId> or <templateId> elements, but before all others in the entity where it is used:
<playingEntity classCode='ENT' determinerCode='INSTANCE'> <sdtc:id root='1.3.6.4.1.4.1.2835.2' extension='EntityID'/> : . </playingEntity>
Patient Identifier
There is a need to record the identifer by which a patient is known to another healthcare provider. This extension provides a role link between the assigned, related or associated entity, and the patient role.
Use of this extension to record the identifier under which the patient is known to a provider is shown below.
<assignedEntity>
<id extension='1' root='1.3.6.4.1.4.1.2835.1'/>
|
The <patient> element records the link between the related, assigned or associated entity and the patient. The <id> element provides the identifier for the patient. The root attribute of the <id> should be the namespace used for patient identifiers by the entity. The extension attribute of the <id> element shall be the patient's medical record number or other identifier used by the entity to identify the patient.