Difference between revisions of "Laboratory Analytical Workflow Profile"
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | The LAW profile addresses the exchange of information related to patient and QC test orders & results between IVD testing systems and health informatics systems. | + | {| style="color: blue;" |
| + | | '''This profile is part of the Pathology and Laboratory Medicine (PaLM) domain, which merged the former AP and LAB domains since 2016, January 4th.''' | ||
| + | |} | ||
| + | The '''Laboratory Analytical Workflow (LAW) profile''' addresses the exchange of information related to patient and QC test orders & results between IVD testing systems and health informatics systems. | ||
__TOC__ | __TOC__ | ||
| Line 12: | Line 15: | ||
==Benefits== | ==Benefits== | ||
| − | + | The LAW profile represents a big step forward to meet the clinical laboratory’s need for stable interoperability of IVD analyzers, by providing IVD analyzer and software vendors with a reproducible and maintainable implementation framework. | |
==Details== | ==Details== | ||
| − | + | This LAW Profile covers the workflow of “Analytical Work Order Steps” (AWOS) between an Analyzer Manager application (e.g., a LAS or a LIS) and an Analyzer (an IVD device). This workflow handles the processing of IVD tests by the Analyzer, on specimen materials. Both | |
| + | patient and quality control (QC) specimens are in scope. | ||
| − | + | All specialties of clinical laboratories (including blood bank testing) are in scope. | |
| + | |||
| + | Tests performed on the point of care by the ward staff or the patient are out of scope of this profile, and addressed by the LPOCT profile instead. | ||
| + | |||
| + | An AWOS is an analytical service to be performed by an analyzer on a specimen. The AWOS is ordered by means of a code representing this analytical service. The code may represent an elementary test (e.g., “measure the glucose level of the specimen”) or a panel of several | ||
| + | elementary tests. In all cases the analytical service is expected to produce observations on the specimen that will be reported back by the Analyzer. The AWOS does not say how to perform the analytical service. The electromechanical handling of an analyzer is out of scope of this profile. | ||
| + | |||
| + | The use cases related to patient specimens are summarized on this diagram: | ||
| + | |||
| + | [[File:LAWUSECASES.jpg]] | ||
| − | |||
| − | |||
==Systems Affected== | ==Systems Affected== | ||
| − | |||
| − | * | + | * In vitro diagnostic devices usually implement the Analyzer Actor of the profile. They obtain analytical work order steps (AWOS) related to a specimen-in-container and perform the requested tests and send back the results. |
| − | * | + | * A middleware or a LIS may implement the Analyzer Manager Actor. It will provide the AWOS to the intended Analyzer and collect the results from those AWOS. |
| − | + | ||
'''Actors & Transactions:''' | '''Actors & Transactions:''' | ||
| − | + | [[File:LAW_ActorsTransac.jpg]] | |
==Specification== | ==Specification== | ||
| Line 43: | Line 53: | ||
'''Underlying Standards:''' | '''Underlying Standards:''' | ||
| − | |||
| − | |||
:* [http://www.hl7.org HL7 2.5.1 + some pre-adopted features of 2.8 & 2.9] | :* [http://www.hl7.org HL7 2.5.1 + some pre-adopted features of 2.8 & 2.9] | ||
| Line 85: | Line 93: | ||
[[Category:Profiles]] | [[Category:Profiles]] | ||
| + | [[Category:PaLM Profile]] | ||
| + | [[Category:HL7v2]] | ||
Latest revision as of 05:32, 1 August 2017
| This profile is part of the Pathology and Laboratory Medicine (PaLM) domain, which merged the former AP and LAB domains since 2016, January 4th. |
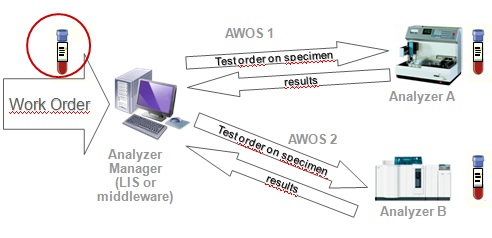
The Laboratory Analytical Workflow (LAW) profile addresses the exchange of information related to patient and QC test orders & results between IVD testing systems and health informatics systems.
Summary
The purpose of the LAW profile is to improve interoperability between IVD testing systems and health informatics systems by reducing complexity and variability in the exchange of information related to patient and QC test orders and to the result thereof. This profile is thus focused on the analytical workflow in the work area of a clinical laboratory. Its Transactions streamline the scheduling, performance and result reporting of Analytical Work Order Steps (AWOS) between IVD testing systems and healthcare IT systems (LIS or middlewares.
Benefits
The LAW profile represents a big step forward to meet the clinical laboratory’s need for stable interoperability of IVD analyzers, by providing IVD analyzer and software vendors with a reproducible and maintainable implementation framework.
Details
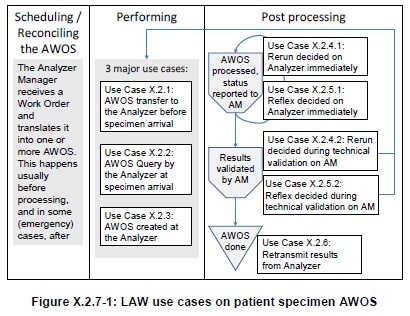
This LAW Profile covers the workflow of “Analytical Work Order Steps” (AWOS) between an Analyzer Manager application (e.g., a LAS or a LIS) and an Analyzer (an IVD device). This workflow handles the processing of IVD tests by the Analyzer, on specimen materials. Both patient and quality control (QC) specimens are in scope.
All specialties of clinical laboratories (including blood bank testing) are in scope.
Tests performed on the point of care by the ward staff or the patient are out of scope of this profile, and addressed by the LPOCT profile instead.
An AWOS is an analytical service to be performed by an analyzer on a specimen. The AWOS is ordered by means of a code representing this analytical service. The code may represent an elementary test (e.g., “measure the glucose level of the specimen”) or a panel of several elementary tests. In all cases the analytical service is expected to produce observations on the specimen that will be reported back by the Analyzer. The AWOS does not say how to perform the analytical service. The electromechanical handling of an analyzer is out of scope of this profile.
The use cases related to patient specimens are summarized on this diagram:
Systems Affected
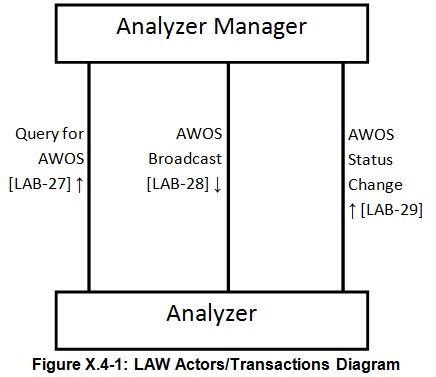
- In vitro diagnostic devices usually implement the Analyzer Actor of the profile. They obtain analytical work order steps (AWOS) related to a specimen-in-container and perform the requested tests and send back the results.
- A middleware or a LIS may implement the Analyzer Manager Actor. It will provide the AWOS to the intended Analyzer and collect the results from those AWOS.
Actors & Transactions:
Specification
Profile Status: Trial Implementation
Documents:
last revision from October 2, 2012
Underlying Standards:
See Also
Related Profiles
No profile depends upon LAW. However it is important to stress the repartition between LAW and LDA: LDA covers laboratory devices involved in the pre-analytical and post-analytical workflows.
LAW covers laboratory IVD devices involved in the analytical workflow.
A middleware system in a laboratory typically plays the role of Automation Manager in LTW and LDA and the role of Analyzer Manager in LAW.
<The following sections can be left out if there is nothing to point to. This is just to show where such information can go.>
Consumer Information
The Profile FAQ Template answers typical questions about what the Profile does. <Replace the link with a link to the actual FAQ page for the Profile>
The Profile Purchasing Template describes considerations when purchasing equipment to deploy this Profile. <Replace the link with a link to the actual Purchasing page for the Profile>
Implementer Information
The Profile Implementation Template provides additional information about implementing this Profile in software. <Replace the link with a link to the actual Implementation page for the Profile>
Reference Articles
<List References (good and bad) (with link if possible) to Journal Articles that mention IHE's work (and hopefully include some analysis). Go ahead, Google: IHE <Profile Name> abstract or Google: IHE <Profile Name> and under the "more" select "Scholar". You might be surprised. >
This page is based on the Profile Overview Template