Difference between revisions of "Laboratory"
| (33 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[ | + | |
| + | {| style="color: red;" | ||
| + | | '''WARNING: As of January 4, 2016 this page is deprecated and superseded by the [[Pathology and Laboratory Medicine (PaLM)]] domain page''' | ||
| + | |} | ||
| + | <br/><br/> | ||
| + | '''IHE Laboratory Domain''' addresses information sharing and workflow related to in vitro diagnostic testing in clinical laboratories as well as point of care testing. The IHE Laboratory Domain was established in 2003 and manages the [[Profiles#IHE Laboratory Profiles| Laboratory Profiles]] and the [[Frameworks#IHE Laboratory Technical Framework| Laboratory Technical Framework]]. We are actively soliciting qualified experts from the healthcare profession and healthcare IT industry for ongoing maintenance, new development, and deployment of domain profiles. | ||
<br/> | <br/> | ||
<br/> | <br/> | ||
| + | |||
| + | {| style="width:100%" border="1" cellpadding="1" | ||
| + | ! '''Special Announcement''' | ||
| + | |In thinking strategically of future work and profiles for IHE Lab and IHE AP, and in the spirit of further collaboration, the co-chairs from IHE AP, IHE Lab, and the IHE Lab/AP Secretariat, recommend a consolidation of the two working groups into one group. We think a merger is beneficial to both groups, as well as being a streamlined process that the Board of IHE International will appreciate. We already are starting to see a merged effort through the “Laboratory Device Automation” reengineering project that has software vendors involved from both domains. We anticipate the future will continue to see a blurring between the two domains and want to be positioned to best address emerging needs. The new domain could be called the “ IHE Pathology and Laboratory Medicine” domain or something similar to reflect all aspects of the laboratory. We also suggest that all co-chairs be retained to assist in leading discussions focused on one area or another and to continue on work already in progress. | ||
| + | In anticipation of this merger, we recommend that an additional day be added to the already planned IHE Lab meeting in Paris from May 19-21. The joint IHE AP/Lab meeting then will be from Tuesday 19th 9am to Friday 22nd at noon. Please let us know what you think of this recommendation. We will be posting logistics for the May meeting in Paris soon. | ||
| + | |||
| + | Sincerely, | ||
| + | IHE AP co-chairs, IHE Lab co-chairs, and IHE AP/LAB Secretariat | ||
| + | |} | ||
| + | |||
| + | |||
{|align=right | {|align=right | ||
| Line 43: | Line 59: | ||
* [[Laboratory_Code_Sets_Distribution | LCSD]] - Laboratory Code Sets Distribution distributes managed sets of clinical laboratory codes (battery, test and observation codes). | * [[Laboratory_Code_Sets_Distribution | LCSD]] - Laboratory Code Sets Distribution distributes managed sets of clinical laboratory codes (battery, test and observation codes). | ||
* [[Inter_Laboratory_Workflow | ILW]] - Inter Laboratory Workflow supports the workflow of orders and results with a subcontracting laboratory. | * [[Inter_Laboratory_Workflow | ILW]] - Inter Laboratory Workflow supports the workflow of orders and results with a subcontracting laboratory. | ||
| − | + | * [[Laboratory_Analytical_Workflow | LAW]] - Laboratory Analytical Workflow supports the workflow of analytical work order steps (AWOS) between an Analyzer Manager and an Analyze (IVD automated device). | |
<br/><br/> | <br/><br/> | ||
| − | == Timeline : | + | == Timeline : 2014-2015 Planning and Development Cycle == |
| − | The following table outlines the activities and steps planned for the | + | The following table outlines the activities and steps planned for the 2014-2015 year. |
{| style="width:100%" border="1" cellpadding="3" | {| style="width:100%" border="1" cellpadding="3" | ||
| Line 56: | Line 72: | ||
! Location | ! Location | ||
|- | |- | ||
| − | | colspan="4" align="center" style="background:#dddddd" | ''' | + | | colspan="4" align="center" style="background:#dddddd" | '''2015''' |
|- | |- | ||
| Year long | | Year long | ||
| LAB TF maintenance and build of new profiles | | LAB TF maintenance and build of new profiles | ||
| − | | Monthly, 10-11 am (CET) | + | | Monthly, second Tuesday of the month 10-11 am (CET) |
| conf calls + gotoMeeting | | conf calls + gotoMeeting | ||
|- | |- | ||
| Jan | | Jan | ||
| NA Connectathon | | NA Connectathon | ||
| − | | Jan | + | | Jan 26-30 |
| − | | | + | | Cleveland, USA |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| April | | April | ||
| EU Connectathon | | EU Connectathon | ||
| − | | April | + | | April 20-24 |
| − | | | + | | Luxembourg |
|- | |- | ||
| − | | | + | | May |
| − | | | + | | Lab Committee Face to face |
| − | | | + | | May 19-22 |
| − | | | + | | Paris, France |
|- | |- | ||
| June | | June | ||
| − | | | + | | Publish Lab TF v6.0 |
| − | + | | | |
| − | + | | | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
|- | |- | ||
| − | | | + | | September |
| Japan Connectathon | | Japan Connectathon | ||
| − | | | + | | |
| − | | | + | | Yokohama, Japan |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
|- | |- | ||
| + | | September | ||
| + | | Publish LCC as supplement for comment? | ||
| | | | ||
| | | | ||
| + | |- | ||
| + | | November | ||
| + | | Lab Committee Face to face | ||
| + | | Japan | ||
| | | | ||
| − | | | + | |- |
|} | |} | ||
| Line 122: | Line 122: | ||
The IVD Industry Connectivity Consortium (IICC) is an organization founded by the top worldwide IVD manufacturers to catalyze worldwide modernization of standards to reduce the costs and inefficiencies associated with device interfacing. Founding members of IICC include leading IVD manufacturers (Abbott Diagnostics, Beckman Coulter, Becton Dickinson, bioMerieux, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare) and two IT companies (Data Innovations and Systelab Technologies). Subsequent to the founding of IICC, the following companies have become full members: Orchard Software (IT). IICC believes that IHE’s LDA integration profile represents the best starting point for defining a worldwide profile, and recommends commissioning a project to extend this integration profile. The IHE Laboratory Domain approved moving forward with the IICC Profile Proposal for the next generation Laboratory Device Automation for the 2010-2011 cycle. This wiki will document this progress. | The IVD Industry Connectivity Consortium (IICC) is an organization founded by the top worldwide IVD manufacturers to catalyze worldwide modernization of standards to reduce the costs and inefficiencies associated with device interfacing. Founding members of IICC include leading IVD manufacturers (Abbott Diagnostics, Beckman Coulter, Becton Dickinson, bioMerieux, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare) and two IT companies (Data Innovations and Systelab Technologies). Subsequent to the founding of IICC, the following companies have become full members: Orchard Software (IT). IICC believes that IHE’s LDA integration profile represents the best starting point for defining a worldwide profile, and recommends commissioning a project to extend this integration profile. The IHE Laboratory Domain approved moving forward with the IICC Profile Proposal for the next generation Laboratory Device Automation for the 2010-2011 cycle. This wiki will document this progress. | ||
| − | [[Laboratory Analytical Workflow|Laboratory Analytical Workflow (LAW)]] | + | [[Laboratory Analytical Workflow|Laboratory Analytical Workflow (LAW)]] A new release was published in 2013 |
| + | <br/><br/> | ||
| + | |||
| + | |||
| + | Currently working on the (LCC) - standardizing how to indicate suggested replacement orders from the filler side and requesting follow up work by the placer. More information can be found on the wiki | ||
| + | [[LCC Long Proposal - wiki|Laboratory-Clinical Communications(LCC)]] | ||
<br/><br/> | <br/><br/> | ||
| Line 203: | Line 208: | ||
| 2012.09.20 | | 2012.09.20 | ||
| Webinar | | Webinar | ||
| + | | | ||
| [https://ihe.webex.com/ihe/lsr.php?AT=pb&SP=EC&rID=62046952&rKey=7af714e14223fa36 Webex Replay] | | [https://ihe.webex.com/ihe/lsr.php?AT=pb&SP=EC&rID=62046952&rKey=7af714e14223fa36 Webex Replay] | ||
|- | |- | ||
| 2013.07.29 | | 2013.07.29 | ||
| Houston, TX | | Houston, TX | ||
| − | | IICC Booth at AACC to Showcase LAW Benefits and IHE-based testing Infrastructure | + | | IICC Booth at AACC to Showcase LAW Benefits and IHE-based testing Infrastructure for LAW |
| [http://ivdconnectivity.org IICC Website] | | [http://ivdconnectivity.org IICC Website] | ||
|} | |} | ||
| Line 218: | Line 224: | ||
!Date!!Organization!!Location!!More Information | !Date!!Organization!!Location!!More Information | ||
|- | |- | ||
| − | | | + | |2015.January 18-23||HL7 (Work Group Meetings)||San Antonio, TX||[http://www.hl7.org/events/workgroupmeetings.cfm?ref=nav Website] |
|- | |- | ||
| − | | | + | |2015.February 12-13||LOINC (Public Clinical LOINC Tutorial & Committee Meeting)||Salt Lake City, UT||[http://loinc.org/meetings/20150212/public-clinical-loinc-tutorial-committee-meeting-02-12-15-02-13-14.ics/view] |
|- | |- | ||
| − | | | + | |2015.March TBD||CLSI Leadership Conference||Arlington, VA||[http://www.cvent.com/events Website] |
|- | |- | ||
| − | | | + | |2015.April 26 - 29||IHTSDO Business Meeting||Copenhagen, Denmark||[http://www.ihtsdo.org/participate/attend-ihtsdo-events] |
|- | |- | ||
| − | | | + | |2015.May 10-15||HL7 (Work Group Meetings)||Paris, France|| [http://www.hl7.org/events/workgroupmeetings.cfm?ref=nav Website] |
| − | |- | + | |- |
| − | | | + | |2015.June 3-4||LOINC (Public Clinical LOINC Tutorial & Committee Meeting)||Salt Lake City, UT||[http://loinc.org/meetings/20150603/public-laboratory-loinc-workshop-committee-meeting-06-03-15-06-04-15.ics/view] |
|- | |- | ||
| − | | | + | |2015.July 26-30||AACC||Atlanta, GA||[https://www.aacc.org/meetings-and-events/annual-meeting/2015-annual-meeting-and-expo] |
|- | |- | ||
| − | | | + | |2015.October 4-7||CAP Annual Meeting||Nashville, TN||[http://www.thepathologistsmeeting.org/] |
|- | |- | ||
| − | | | + | |2015.October 4-9||HL7 Annual Plenary|| Atlanta, GA||[http://www.hl7.org/ Website] |
|- | |- | ||
| − | | | + | |2015.October TBD||Pathology Informatics|| Chicago, IL||[http://www.pathinformatics.pitt.edu/ Website] |
|- | |- | ||
| − | | | + | |2015.October 25-30||IHTSDO ||Montevideo, Uruguay|| [http://www.ihtsdo.org/participate/attend-ihtsdo-events] |
|- | |- | ||
| − | | | + | |2015.October 28 - 30||ASCP Annual Meeting||Long Beach, CA||[http://www.ascp.org/2015-Annual-Meeting/index.html] |
|- | |- | ||
| − | | | + | |2015.November 14 - 18||AMIA Annual Symposium||San Francisco, CA||[https://www.amia.org/amia2015] |
|- | |- | ||
| − | | | + | | |
| − | + | |-} | |
| − | | | ||
| − | |||
<br/><br/> | <br/><br/> | ||
Latest revision as of 15:04, 15 December 2015
| WARNING: As of January 4, 2016 this page is deprecated and superseded by the Pathology and Laboratory Medicine (PaLM) domain page |
IHE Laboratory Domain addresses information sharing and workflow related to in vitro diagnostic testing in clinical laboratories as well as point of care testing. The IHE Laboratory Domain was established in 2003 and manages the Laboratory Profiles and the Laboratory Technical Framework. We are actively soliciting qualified experts from the healthcare profession and healthcare IT industry for ongoing maintenance, new development, and deployment of domain profiles.
| Special Announcement | In thinking strategically of future work and profiles for IHE Lab and IHE AP, and in the spirit of further collaboration, the co-chairs from IHE AP, IHE Lab, and the IHE Lab/AP Secretariat, recommend a consolidation of the two working groups into one group. We think a merger is beneficial to both groups, as well as being a streamlined process that the Board of IHE International will appreciate. We already are starting to see a merged effort through the “Laboratory Device Automation” reengineering project that has software vendors involved from both domains. We anticipate the future will continue to see a blurring between the two domains and want to be positioned to best address emerging needs. The new domain could be called the “ IHE Pathology and Laboratory Medicine” domain or something similar to reflect all aspects of the laboratory. We also suggest that all co-chairs be retained to assist in leading discussions focused on one area or another and to continue on work already in progress.
In anticipation of this merger, we recommend that an additional day be added to the already planned IHE Lab meeting in Paris from May 19-21. The joint IHE AP/Lab meeting then will be from Tuesday 19th 9am to Friday 22nd at noon. Please let us know what you think of this recommendation. We will be posting logistics for the May meeting in Paris soon. Sincerely, IHE AP co-chairs, IHE Lab co-chairs, and IHE AP/LAB Secretariat |
|---|
The IHE Laboratory Domain is sponsored by:
- College of American Pathologists (CAP)
- JAHIS
- ASIP Santé (The French Agency of Shared Healthcare Information Systems)
Previous sponsors include:
- Groupement pour la Modernisation du Système d'Information Hospitalier (GMSIH), elapsed
- Société d'Informatique de Laboratoire (SFIL)
- IHE Japan
- Quick Links
- Laboratory Planning Committee
- Laboratory Technical Committee
- Laboratory Meeting Roster
- Laboratory Profiles
- Laboratory Technical Framework
- IHE Laboratory Committee Google Group
- Laboratory Strategic Priorities
Domain Profiles
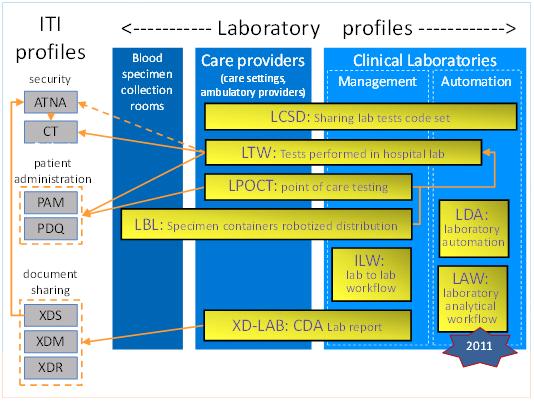
- LTW - Laboratory Testing Workflow integrates ordering and performance of in-vitro diagnostic tests by a clinical laboratory inside a healthcare institution.
- XD-LAB - Sharing Laboratory Reports describes the content (human and machine readable) of an electronic clinical laboratory report.
- LDA - Laboratory Device Automation integrates an Automation Manager and robotic laboratory equipment (pre-analytical devices, analyzers, post-analytical devices) in a clinical lab.
- LBL - Laboratory Barcode Labeling integrates robotic specimen container labeling systems with sources of order-related labelling information.
- LPOCT - Laboratory Point Of Care Testing integrates performing and collecting the results of in-vitro testing at the point of care or patient’s bedside.
- LCSD - Laboratory Code Sets Distribution distributes managed sets of clinical laboratory codes (battery, test and observation codes).
- ILW - Inter Laboratory Workflow supports the workflow of orders and results with a subcontracting laboratory.
- LAW - Laboratory Analytical Workflow supports the workflow of analytical work order steps (AWOS) between an Analyzer Manager and an Analyze (IVD automated device).
Timeline : 2014-2015 Planning and Development Cycle
The following table outlines the activities and steps planned for the 2014-2015 year.
| Timeframe | Activity | Scheduled | Location |
|---|---|---|---|
| 2015 | |||
| Year long | LAB TF maintenance and build of new profiles | Monthly, second Tuesday of the month 10-11 am (CET) | conf calls + gotoMeeting |
| Jan | NA Connectathon | Jan 26-30 | Cleveland, USA |
| April | EU Connectathon | April 20-24 | Luxembourg |
| May | Lab Committee Face to face | May 19-22 | Paris, France |
| June | Publish Lab TF v6.0 | ||
| September | Japan Connectathon | Yokohama, Japan | |
| September | Publish LCC as supplement for comment? | ||
| November | Lab Committee Face to face | Japan | |
Current Activity
The IVD Industry Connectivity Consortium (IICC) is an organization founded by the top worldwide IVD manufacturers to catalyze worldwide modernization of standards to reduce the costs and inefficiencies associated with device interfacing. Founding members of IICC include leading IVD manufacturers (Abbott Diagnostics, Beckman Coulter, Becton Dickinson, bioMerieux, Ortho Clinical Diagnostics, Roche Diagnostics, and Siemens Healthcare) and two IT companies (Data Innovations and Systelab Technologies). Subsequent to the founding of IICC, the following companies have become full members: Orchard Software (IT). IICC believes that IHE’s LDA integration profile represents the best starting point for defining a worldwide profile, and recommends commissioning a project to extend this integration profile. The IHE Laboratory Domain approved moving forward with the IICC Profile Proposal for the next generation Laboratory Device Automation for the 2010-2011 cycle. This wiki will document this progress.
Laboratory Analytical Workflow (LAW) A new release was published in 2013
Currently working on the (LCC) - standardizing how to indicate suggested replacement orders from the filler side and requesting follow up work by the placer. More information can be found on the wiki
Laboratory-Clinical Communications(LCC)
How to Participate
As a Laboratorian, Pathologist, or Advocate
Get involved in the planning committee. This is where the clinical problems are discussed and prioritized. The planning committee is an open group and is interested in having your input. Bring us your interoperability problems. You can also help by participating in the HIMSS showcase scenario definitions.
You can do this by:
- Request to join our IHE Laboratory Committee Google Group
- Attend telephone conferences and face to face meetings
- Submit new profile proposals
As a Health IT Implementer
Get involved in the technical committee. Help make sure profiles are feasible and will work for you. Implement the profile, and come experience the IHE Connectathon event. 2011 Connectathon video clip
As a Health IT Buyer
In addition to getting involved in the planning committee, hold your vendors accountable. Put a statement in your RFP that indicates you want the vendor to support the actor in the profile you desire.
Demonstrations & Presentations
The following are recent and upcoming public presentations and demonstrations about IHE Lab. Email our IHE Laboratory Google Group about presentations or demonstrations you know about.
| Date | Location | Event | Details |
|---|---|---|---|
| 2010.07.20 | Webinar | IHE Education Webinar Series Laboratory Domain (REPLAY available) | 2010 IHE Webinar Schedule |
| 2010.11.12 | Netherland | IHE Netherlands organizes on November 12, 2010 its seventh annual conference in Spant! at Bussum. | www.ihe-nl.org |
| 2011.02.20 | Orlando, FL | Interoperability Showcase, HIMSS Annual Conference and Exposition | www.himssconference.org |
| 2011.03.16 | Netherland | Care & ICT from 16 to 18 March 2011. | www.ihe-nl.org |
| 2011.08.22 | Atlanta, GA | Interoperability Showcase, Public Health Informatics Conference | www.cdc.gov/phiconference |
| 2011.11.08 | Paris | Journées internationales de biologie (JIB) - Presentation of LAW profile | JIB 2011 |
| 2011.12.06 | Paris | Pre-connectathon meeting of IHE France - Presentation of LAW profile and LBL new option | IHE France |
| 2012.04.02 | London | 22nd European Congress of Clinical Microbiology and Infectious Diseases | EECMID 2012 Presentation |
| 2012.09.20 | Webinar | Webex Replay | |
| 2013.07.29 | Houston, TX | IICC Booth at AACC to Showcase LAW Benefits and IHE-based testing Infrastructure for LAW | IICC Website |
Standards Organization - Professional Meetings/Conferences
See Also
Standard Organizations Link List
This page is based on the Domain Template.
| Date | Organization | Location | More Information |
|---|---|---|---|
| 2015.January 18-23 | HL7 (Work Group Meetings) | San Antonio, TX | Website |
| 2015.February 12-13 | LOINC (Public Clinical LOINC Tutorial & Committee Meeting) | Salt Lake City, UT | [1] |
| 2015.March TBD | CLSI Leadership Conference | Arlington, VA | Website |
| 2015.April 26 - 29 | IHTSDO Business Meeting | Copenhagen, Denmark | [2] |
| 2015.May 10-15 | HL7 (Work Group Meetings) | Paris, France | Website |
| 2015.June 3-4 | LOINC (Public Clinical LOINC Tutorial & Committee Meeting) | Salt Lake City, UT | [3] |
| 2015.July 26-30 | AACC | Atlanta, GA | [4] |
| 2015.October 4-7 | CAP Annual Meeting | Nashville, TN | [5] |
| 2015.October 4-9 | HL7 Annual Plenary | Atlanta, GA | Website |
| 2015.October TBD | Pathology Informatics | Chicago, IL | Website |
| 2015.October 25-30 | IHTSDO | Montevideo, Uruguay | [6] |
| 2015.October 28 - 30 | ASCP Annual Meeting | Long Beach, CA | [7] |
| 2015.November 14 - 18 | AMIA Annual Symposium | San Francisco, CA | [8] |