Difference between revisions of "Cancer Registry Pathology Report"
| (12 intermediate revisions by 2 users not shown) | |||
| Line 9: | Line 9: | ||
==Glossary== | ==Glossary== | ||
| − | + | ;International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3)<br> | |
| + | Classification system for reporting incidences of malignant diseases. | ||
| + | |||
| + | ;NAACCR | ||
| + | The North American Association of Central Cancer Registries. A collaborative umbrella organization for cancer registries, governmental agencies, professional organizations, and private groups in North America interested in enhancing the quality and use of cancer registry data. | ||
| + | |||
| + | ;Pathology Report <br> | ||
| + | The written description of the microscopic examination of a tissue. The gross description reports the physical characteristics of the tissue: size, color, and abnormalities visible with the unaided eye. The microscopic description reports the cellular characteristics aided by the use of a microscope: what cells are involved, the behavior, and the aggressiveness or grade of any abnormality. The final diagnosis is a summary of the findings and indicates the pathologist’s impression of what was found in concise terms. | ||
==Issue Log== | ==Issue Log== | ||
| Line 20: | Line 27: | ||
# Issue: Grouping: Does this need to be modified for the CRC profile? | # Issue: Grouping: Does this need to be modified for the CRC profile? | ||
#* Yes, not sure how | #* Yes, not sure how | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{Fixme|Register Pathology Implementation Guide with HL7 Message Profile Registry}} | {{Fixme|Register Pathology Implementation Guide with HL7 Message Profile Registry}} | ||
| Line 44: | Line 46: | ||
=Volume I= | =Volume I= | ||
<pre>Add the following bullet to the list of profiles</pre> | <pre>Add the following bullet to the list of profiles</pre> | ||
| − | * The Cancer Registry Content Profile ( | + | * The Cancer Registry Pathology Report Content Profile (CPR) defines the the content used in the {{ILink|Cancer Registry Content|PCC-11}} to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message. |
===Dependencies=== | ===Dependencies=== | ||
<pre>Add the following row(s) to the list of dependencies</pre> | <pre>Add the following row(s) to the list of dependencies</pre> | ||
| − | {| | + | {{T|Integration Profile|Dependency|Dependency Type|Purpose|Rows= |
| − | + | {{R|Cancer Registry Pathology Report (CPR)|Care Management (CM)|The Content Creator actor of the CPR profile must be grouped with the Clinical Data Source Actor of the CM profile|The CPR profile defines the content sent in the PCC-11 transaction specified in the CM profile}} | |
| − | + | {{R|Cancer Registry Pathology Report (CPR)|Care Management (CM)|The Content Consumer actor of the CPR profile must be grouped with the Care Manager Actor of the CM profile|The CPR profile defines the content recieved in the PCC-11 transaction specified in the CM profile}} | |
| − | + | {{R|Cancer Registry Pathology Report (CPR)|ATNA|Actors the CPR profile shall implement the Secure Node Actor of the ATNA profile|Ensures that transmissions and changes to patient health information are logged in an audit repository, and that communication is secured between nodes.}} | |
| − | + | {{R|Cancer Registry Pathology Report (CPR)|ATNA|Actors the CPR profile shall implement the Time Client Actor of the CT profile|Ensures that concistent time is used in all messages.}} | |
| − | | | + | }} |
| − | |Cancer Registry Pathology Report Content | ||
| − | |Care Management | ||
| − | ATNA | ||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | | | ||
| − | |} | ||
| − | |||
==Cancer Registry Pathology Report Content == | ==Cancer Registry Pathology Report Content == | ||
The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the {{ILink|Cancer Registry Content|PCC-11}} to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message. | The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the {{ILink|Cancer Registry Content|PCC-11}} to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message. | ||
| Line 111: | Line 103: | ||
===Actors/Transaction=== | ===Actors/Transaction=== | ||
There are two actors in this profile, the Content Creator and the Content Consumer. Content is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile. | There are two actors in this profile, the Content Creator and the Content Consumer. Content is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[image:cccc.png|frame|center|Cancer Registry Pathology Report Content Actor Diagram]] | [[image:cccc.png|frame|center|Cancer Registry Pathology Report Content Actor Diagram]] | ||
| − | |||
| − | |||
=== Grouping === | === Grouping === | ||
| − | ==== | + | ==== Care Management (CM) ==== |
| − | + | The [[Content Creator]] of this profile must be grouped with the [[Clinical Data Source]] of the Care Management profile. The Clinical Data Source actor must implement the V2 Care Management Update Option. | |
| − | + | The [[Content Consumer]] Actor of this profile must be grouped with the [[Care Manager]] actor of the Care Management profile. | |
| − | |||
| − | === | + | ==== Basic Patient Privacy Consents (BCCP) ==== |
| − | + | When patient consent is required (for example, as in the case of Quebec), the Content Creator may be grouped with the Content Consumer Actor of the BPPC profile. When grouped with that actor, the Content Creator shall verify that an appropriate consent has been registered for the patient to share the pathology data prior to sending the message. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Policy Considerations == | == Policy Considerations == | ||
| Line 154: | Line 122: | ||
A Content Consumer may require that all reports be transmitted, regardless of relevance to cancer, or it may require transmission to be restricted based on specific selection criteria. This is a policy decision made by the Content Receiver based on state, provincial and fedreal legislations and/or regulations, rules or by operating policy. | A Content Consumer may require that all reports be transmitted, regardless of relevance to cancer, or it may require transmission to be restricted based on specific selection criteria. This is a policy decision made by the Content Receiver based on state, provincial and fedreal legislations and/or regulations, rules or by operating policy. | ||
| − | == Security Considerations | + | == Security Considerations == |
| − | |||
| − | |||
| − | |||
| − | |||
== Requirements/Capabilities == | == Requirements/Capabilities == | ||
| Line 165: | Line 129: | ||
* List of anatomic pathology reports | * List of anatomic pathology reports | ||
* Standards for Identifying Pathology Reports | * Standards for Identifying Pathology Reports | ||
| − | + | * List of Cancer Medicine [http://www.rapidtest.com/products-elisakits.html Elisa kit] | |
; Message Conformance | ; Message Conformance | ||
| − | Message | + | Message can be validated using: <br> |
| − | + | : [http://www.naaccr.org/index.asp?Col_SectionKey=7&Col_ContentID=501] : NAACCR Volume V Messaging Workbench Profile. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | : [http://www.naaccr.org/index.asp?Col_SectionKey=7&Col_ContentID= | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 18:53, 7 December 2009
Introduction
This is a draft of the Cancer Registry Pathology Report Content Profile (CRPR) supplement to the PCC Technical Framework. This draft is a work in progress, not the official supplement or profile.
Profile Abstract
The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Glossary
- International Classification of Diseases for Oncology, 3rd Edition (ICD-O-3)
Classification system for reporting incidences of malignant diseases.
- NAACCR
The North American Association of Central Cancer Registries. A collaborative umbrella organization for cancer registries, governmental agencies, professional organizations, and private groups in North America interested in enhancing the quality and use of cancer registry data.
- Pathology Report
The written description of the microscopic examination of a tissue. The gross description reports the physical characteristics of the tissue: size, color, and abnormalities visible with the unaided eye. The microscopic description reports the cellular characteristics aided by the use of a microscope: what cells are involved, the behavior, and the aggressiveness or grade of any abnormality. The final diagnosis is a summary of the findings and indicates the pathologist’s impression of what was found in concise terms.
Issue Log
Open Issues
- Issue: Dependencies: need help understanding this component
- Depends on Care Management with the V2 option (and by reference ATNA and CT)
- Issue: Modify Actor/Transactions (Keith)
- Issue: Modify Content Module for V2 profiles (Keith)
- Issue: Grouping: Does this need to be modified for the CRC profile?
- Yes, not sure how
![]() -->>Register Pathology Implementation Guide with HL7 Message Profile Registry<<--
-->>Register Pathology Implementation Guide with HL7 Message Profile Registry<<--
- In Progress
Closed Issues
- Issue: Glossary: Use MERP glossary document or consolidated glossary tool?
- Refer to the existing document
- Issue: Options: need help understanding this component
- Won't have any
- Added options based on discussion at May f2f meeting.
- Issue: Transaction Definitions: need help understanding this component
- Not relevant to this profile
- Issue: What information do we need to include in 3.1.2, 3.1.3, 3.2, 3.3, 3.4?
- Just reference the NAACCR Documentation on this section
- Should we include a sample message?
- include a link to a webpage of a sample message. There are ways to convert a document to a webpage....
Volume I
Add the following bullet to the list of profiles
- The Cancer Registry Pathology Report Content Profile (CPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Dependencies
Add the following row(s) to the list of dependencies
| Integration Profile | Dependency | Dependency Type | Purpose |
|---|---|---|---|
| Cancer Registry Pathology Report (CPR) | Care Management (CM) | The Content Creator actor of the CPR profile must be grouped with the Clinical Data Source Actor of the CM profile | The CPR profile defines the content sent in the PCC-11 transaction specified in the CM profile |
| Cancer Registry Pathology Report (CPR) | Care Management (CM) | The Content Consumer actor of the CPR profile must be grouped with the Care Manager Actor of the CM profile | The CPR profile defines the content recieved in the PCC-11 transaction specified in the CM profile |
| Cancer Registry Pathology Report (CPR) | ATNA | Actors the CPR profile shall implement the Secure Node Actor of the ATNA profile | Ensures that transmissions and changes to patient health information are logged in an audit repository, and that communication is secured between nodes. |
| Cancer Registry Pathology Report (CPR) | ATNA | Actors the CPR profile shall implement the Time Client Actor of the CT profile | Ensures that concistent time is used in all messages. |
Cancer Registry Pathology Report Content
The Cancer Registry Pathology Report Content Profile (CRPR) defines the the content used in the PCC-11 to send a completed pathology report to a Cancer Registry using an HL7 Version 2.3.1 ORU message.
Monitoring the occurence of cancer is a cornerstone of cancer control decision making. this monitoring, referred to as cancer surveillance, can be used to trigger case investigations, follow trends, evaluate the effectiveness of prevention measures such as screening and early detection programs and suggest public health priorities. It is vital to identify and registrar all cancer cases. By not identifying all of the cancer cases, cancer incidence will be underestimated, giving a false impression of the magnitude of the cancer problem in the registry’s population area. Inaccurate incidence rates can misdirect cancer control efforts, and provide a false picture of the effectiveness of treatment efforts.
Because most cancers are definitively diagnosed by microscopic examination of tissue, cancer surveillance programs rely on pathology reports to identify new cases and collect further information on cases previously reported.
Two challenges relate to pathology laboratory reporting of cancer cases. Laboratories may photocopy selected pathology reports and mail them to the cancer registry. This method is labor intensive and prone to missing cases because the laboratory staff is not aware of the full case definition for identifying a cancer case. Additionally, the cancer registry staff must re-enter the information into the cancer registry with a risk of data entry errors.
Alternately, laboratories may choose not to actively report cases, but allow cancer registry personnel to identify and photocopy appropriate pathology reports on site. This is very costly for the registry and has the same risk of data entry errors when the reports are manually entered into the registry database.
Further information on the benefits, challenges and the cancer registry's uses of electronic pathology reporting can be found in the:
- [4] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
Use Cases
- Pre-condition
A woman goes in for a routine mammogram and a suspicious area is noted. The woman is referred by her family physician to a surgeon who does a biopsy and sends the tissue sample to the pathology laboratory for review and diagnosis.
The pathology laboratory receives the specimen, logs it into the laboratory information system (LIS) and prepares the specimen for analysis. The pathologist analyzes the specimen and dictates his/her findings, which are then transcribed into the LIS. The pathologist verifies the accuracy of the report and signs the transcribed report. The LIS marks the pathology report as "Final", triggering the use case events.
- Events
- 1. Pathology Laboratory Creates Message
The LIS identifies that the report matches submission criteria because it is a histopathology test (biopsy) and the diagnosis relates to cancer ("carcinoma"). The LIS gathers information from its database and other appropriate databases to create a message that contains all of the required data elements. The LIS validates the data to ensure it conforms to the HL7 2.3.1 ORU-RO1 specification and transmits it to the Cancer Registry using a secure connection.
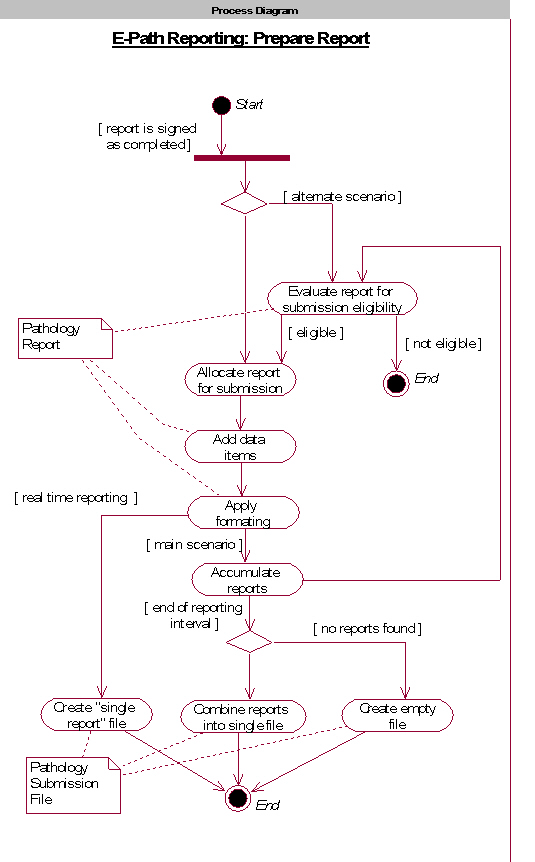
: [1] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
- 1. Cancer Registry Processes Registration
The Cancer Registry receives the HL7 messages, parses it into the processing database and determines that a cancer diagnosis has been documented. The Cancer Registry staff codes the medical information. The pathology data and coded information is exported to the Cancer Registry Database and the message is archived.

: [2] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
- Post-condition
Pathology information has been added to the Cancer Registry Database.
The message is available for future use as needed.
Scope
The following diagram constrains the scope of the Cancer Registry Pathology Report Content Profile.
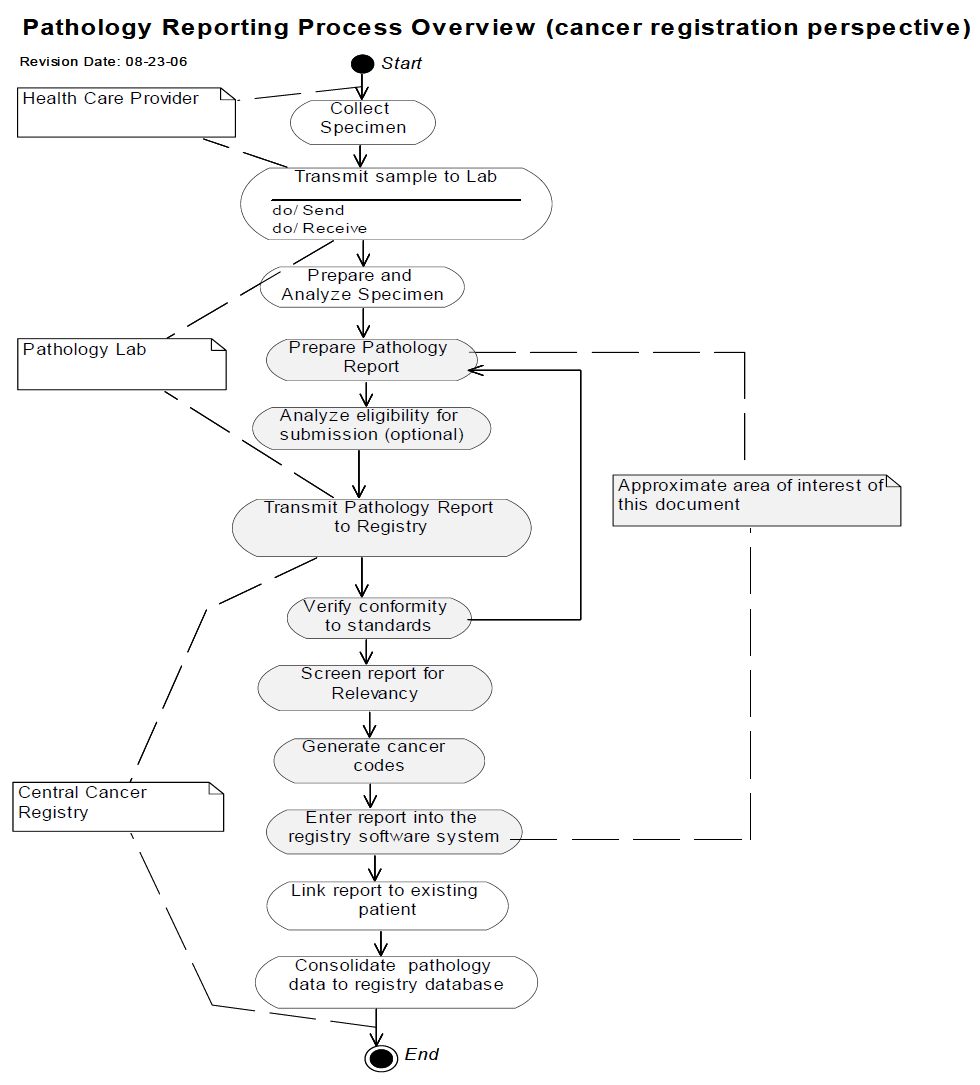
: [3] : North American Association of Central Cancer Registries (NAACCR) Electronic Pathology (E-Path) Reporting Guidelines, Dec 2006.
Actors/Transaction
There are two actors in this profile, the Content Creator and the Content Consumer. Content is created by a Content Creator and is to be consumed by a Content Consumer. The sharing or transmission of content from one actor to the other is addressed by the appropriate use of IHE profiles described below, and is out of scope of this profile.
Grouping
Care Management (CM)
The Content Creator of this profile must be grouped with the Clinical Data Source of the Care Management profile. The Clinical Data Source actor must implement the V2 Care Management Update Option.
The Content Consumer Actor of this profile must be grouped with the Care Manager actor of the Care Management profile.
Basic Patient Privacy Consents (BCCP)
When patient consent is required (for example, as in the case of Quebec), the Content Creator may be grouped with the Content Consumer Actor of the BPPC profile. When grouped with that actor, the Content Creator shall verify that an appropriate consent has been registered for the patient to share the pathology data prior to sending the message.
Policy Considerations
- Batch or Real Time Reporting
Because the Cancer Registry Pathology Report Content Profile relates to secondary use of data, Batch Reporting with a frequency of every 24 hours is recommended.
- Case Selection
A Content Consumer may require that all reports be transmitted, regardless of relevance to cancer, or it may require transmission to be restricted based on specific selection criteria. This is a policy decision made by the Content Receiver based on state, provincial and fedreal legislations and/or regulations, rules or by operating policy.
Security Considerations
Requirements/Capabilities
- Case selection
Provide option to select all reports or only those that meet selection criteria. Refer to Volume II for:
- List of anatomic pathology reports
- Standards for Identifying Pathology Reports
- List of Cancer Medicine Elisa kit
- Message Conformance
Message can be validated using:
- [5] : NAACCR Volume V Messaging Workbench Profile.
