Registry Content Submission-CathPCI
This content profile provides an HL7 CDA Implementation Guide for the content needed to support submissions to the NCDR® CathPCI Registry® v4.4.
Summary
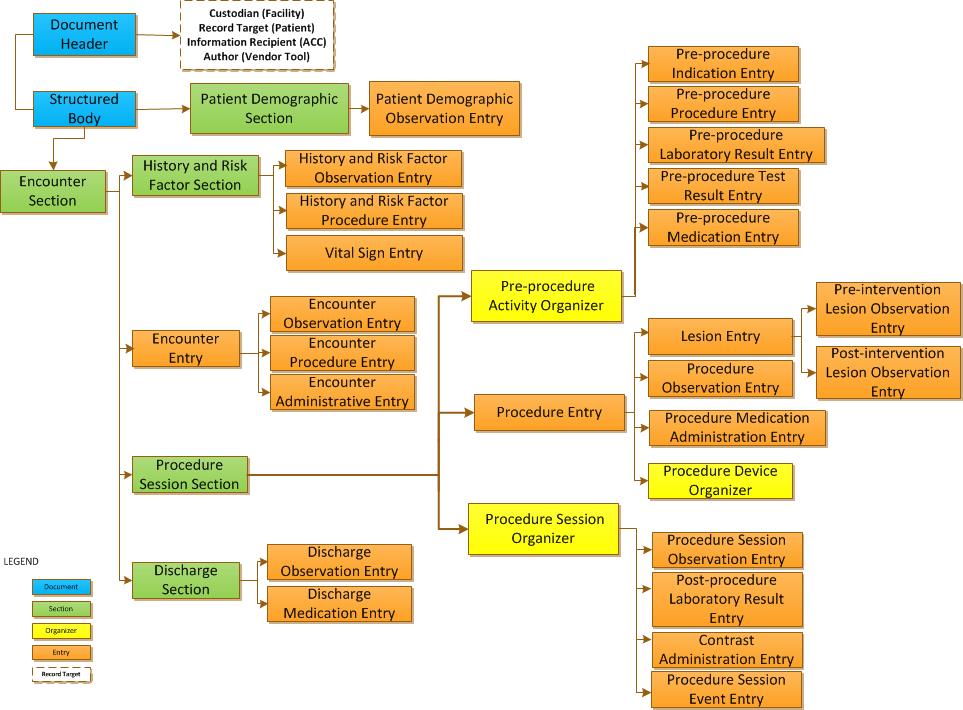
The RCS-C Profile specifies the content structure and value sets for reporting the data elements collected during a cardiac catheterization and/or PCI to the NCDR. The NCDR CathPCI Registry V4.4Coder’s Data Dictionary stipulates the RCS-C profile data and value sets.
Benefits
The Registry Content Submission-CathPCI v4.4 (RCS-C) Profile has been developed as an IHE Technical Framework implementation to provide an industry standard specification for reporting to the NCDR CathPCI Registry v4.4. Use of an industry standard will reduce the reporting burden on submitters by using the same standard mandated for hospital reporting for Meaningful Use, Stage 2. The NCDR Registries help hospitals and private practices measure and improve the quality of cardiovascular care they provide.
Details
The RCS-C profile specifies the content structure and value sets for reporting the data elements collected during a cardiac catheterization and/or PCI to the NCDR. The NCDR CathPCI Registry V4.4Coder’s Data Dictionary stipulates the RCS-C profile data and value sets.
The RSC-C profile supports a single use case, Compile and Transfer RSC-Content (i.e., Submission to a CathPCI Registry). In this use case, the Content Creator gathers the data that is required to create the RSC-C document instance. The role of content creator may be filled by a healthcare facility system that documents the cardiac catheterization and PCI procedures. The Content Creator sends the completed document instance to a Content Consumer. The Content Consumer parses the content and extracts the discrete data.
RCS-C Profile specifies the content structure and value sets for reporting the data elements collected during a cardiac catheterization and/or PCI to the NCDR®
Systems Affected
Specification
Profile Status: Trial Implementation
Documents:
Cardiology Technical Framework:
Underlying Standards:
See Also
Related Profiles
- Cath Report Content [CRC] format for a CDA report of a cardiac Cath/PCI procedure, including discrete data elements.
Consumer Information
The NCDR CathPCI Coder's Data Dictionary defines the elements that are captured for the NCDR CathPCI v4.4 Registry.
Implementer Information
Reference Articles