Pathology and Laboratory Medicine (PaLM)
Origin
The Pathology and Laboratory Medicine (PaLM) domain of IHE becomes active as of January 2016. This domain merges and supersedes the two prior domains Laboratory (LAB) and Anatomic Pathology (AP) respectively launched in 2003 and 2006. The main reason for this merger was the recognition of a significant amount of similarities of the two prior domains scopes, and a long practice of reuse of assets (content modules, transactions) and common thinking from one another. The decision for this merger has been prepared collectively along year 2015 by the LAB and AP leaderships, common secretariat and memberships, and was approved by the Board of IHE International on November 12, 2015.
Scope
The PaLM domain covers:
- the representation and exchange of digital structured data, digital documents, digital images related to ordering, scheduling, performing and reporting diagnostic observations on in-vitro specimens collected from a patient or a non-living subject ;
- the representation and exchange of digital structured data related to specimen management (preparation, transportation, handoff, aliquoting, storage, retrieval)
- the secondary use and exchange of the observation results
- the diagnostic storage and reuse of specimens in bio banks
when a pathology laboratory is involved and in charge of the production of the observation report following the diagnostic/prognostic/screening tests, this laboratory having at least one of the specialties listed below:
| Laboratory specialty | Sub-specialties |
|---|---|
| anatomic pathology specialties | surgical pathology, autopsy, cytopathology, image cytometry, immunohistochemistry |
| clinical pathology specialties | clinical chemistry, hematology, coagulation, blood gas, microbiology, immunology (allergy, auto-immunity, serology), transfusion medicine (blood bank testing), transplant compatibility testing (HLA), fertility, assisted medical procreation, cytogenetic (karyotype, molecular cytogenetic), drug monitoring and toxicology, flow cytometry |
| molecular pathology specialties | gene mutations detection in tumor cells, genetic identification and characterization of infectious agents, diagnostic of genetic disorders |
Sponsorship
The current sponsors of the PaLM domain are:
In their first years of existence the prior domains LAB and/or AP were also sponsored by GMSIH, ADICAP,SEIS, SEAP, SFIL and IHE Japan, ASIP Santé.
Current leadership
- Planning co-chairs: Raj Dash, MD - FCAP ; Riki Merrick
- Technical co-chairs: François Macary, Phast ; Yoshimi Hirasawa
- Secrataries: Mary Kennedy - CAP ; Carolyn Knapik - CAP
Quick links
- PaLM Planning Committee
- PaLM Technical Committee
- PaLM Meeting Roster
- PaLM Profiles
- PaLM Technical Framework
- IHE PaLM Google Group
- PaLM Board Report
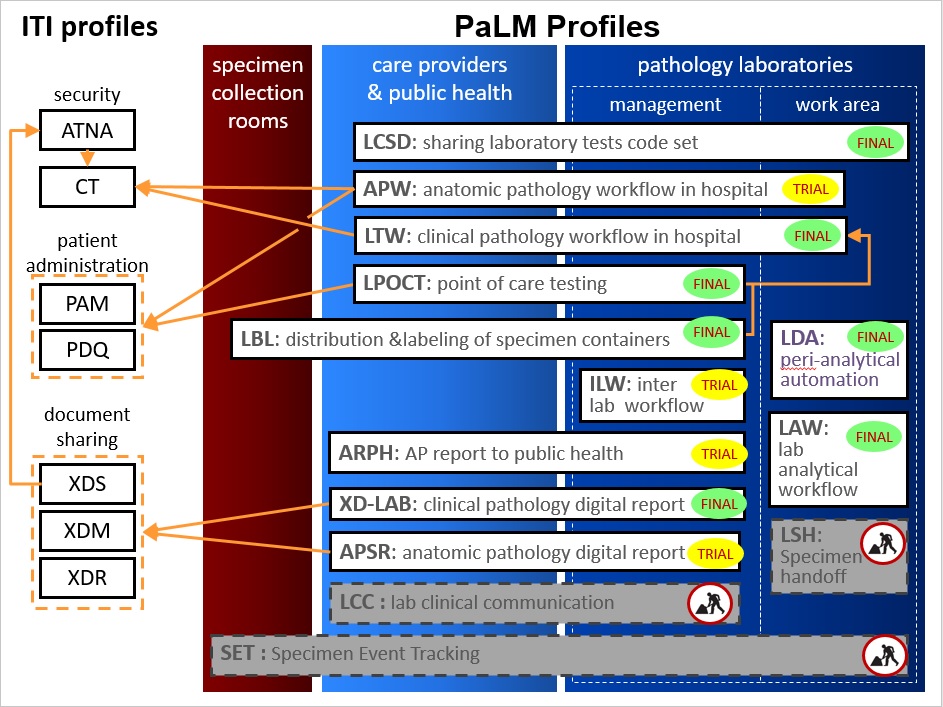
Overview of the PaLM profiles
IT Systems that can leverage PaLM profiles
Non-comprehensive list:
- EHR-S in hospital and ambulatory care settings.
- clinical and/or anatomic pathology laboratory information systems (LIS)
- public health information systems and public health lab information systems
- transfusion medicine systems
- electronic healthcare record shared infrastructures (PHR, HIE …)
- robotic specimen container distributers
- barcode labelers
- robotic specimen transportation systems
- robotic peri-analytical devices in the laboratory work area
- IVD analyzers in laboratory or on the point of care
- middleware systems handling a set of analyzers and/or of peri-analytical devices, in laboratory or on the point of care.
- imaging modalities
- PACS and digital archive systems
- biobank management systems
Guidance material and tools
Timeline: 2016/17 Planning and Development Cycle
The following table outlines the activities and steps planned for the 2016 year.
| Timeframe | Activity | Scheduled | Location |
|---|---|---|---|
| 2017 | |||
| Year long | PaLM TF maintenance and build of new profiles | Monthly, second Wednesday of the month 8-10 am (CET), 1st hour = planning & mgmt, 2nd hour = technical | conf calls + gotoMeeting |
| Jan | NA Connectathon | Jan 23-27 | Cleveland, USA |
| April | EU Connectathon | April 3-7 | Venice, Italy |
| May | PaLM Committee Face to face | May 31 - June 2 | Tokyo, Japan |
| July | Publish PaLM TF v7.0 | July 5th | www.ihe.net |
| September | Japan Connectathon | September TBD | Japan, Tokyo |
| November | PaLM Committee Face to face | November TBD | Sardinia, Italy |
Current Activity
Laboratory Analytical Workflow (LAW) has reached final text status after a session of intensive testing at the January 2016 NA connectathon. This integration profile, which has been built in a joint project between IHE and the IVD Industry Connectivity Consortium (IICC), will also be published in 2016 as the new standard “AUTO16” for analyzers interfaces by the Clinical Laboratory Standards Institute (CLSI).
Currently working on the Laboratory Clinical Communication (LCC) profile - standardizing how to indicate suggested replacement orders from the filler side and requesting follow up work by the placer. More information can be found on the wiki
Laboratory-Clinical Communications(LCC)
Laboratory Specimen Handoff (LSH)
IHE Profile to US Realm Lab Guide Gap Analysis
2016 Proposals:
Specimen Event Tracking (SET) Profile - changes to specimen during processing at all steps from collection, receipt in the lab to storage, including derived material (e.g. slides created from specimen)
SET brief proposal
Structured Reporting (SR) Profile for Clinical Data Capture/Reporting across Multiple Domains - current APSR, using CDA standard, is focused on anatomic pathology, so broaden content; this will be a white paper to explore the current state around data element definitions (SDC, DEX) and how these can be integrated into clinical pathways using business rules, without relying on a specific standard for exchange - requires cross domain collaboration (DCC notified) SR brief proposal
APSR v2.0 - Future release of Anatomic Pathology Structured Report specified on wiki + ART-DECOR
Update APW for Digital Pathology and RAD-16 retrieve Image Modifications - need to be able to support digital pathology - i.e. the storage and retrieval of images created from pathology slides - in collaboration with DICOM WG 26 Digital Pathology brief proposal
Representation of laboratory results in patient summaries using both HL7 CDA and FHIR, LOINC and SNOMED CT - collaboration with IHTSDO, Regenstrief and HL7 - Lab result in patient summaries brief proposal
How to Participate
As a Laboratorian, Pathologist, or Advocate
Get involved in the planning committee. This is where the clinical problems are discussed and prioritized. The planning committee is an open group and is interested in having your input. Bring us your interoperability problems. You can also help by participating in the HIMSS showcase scenario definitions.
You can do this by:
- Becoming a IHE Member Organization
- Request to join our IHE PaLM Google Group
- Attend telephone conferences and face to face meetings
- Submit new profile proposals
As a Health IT Implementer
Get involved in the technical committee. Help make sure profiles are feasible and will work for you. Implement the profile, and come experience the IHE Connectathon event. 2011 Connectathon video clip
As a Health IT Buyer
In addition to getting involved in the planning committee, hold your vendors accountable. Put a statement in your RFP that indicates you want the vendor to support the actor in the profile you desire.
Demonstrations & Presentations
The following are recent and upcoming public presentations and demonstrations about IHE Lab. Email our IHE PaLM Google Group about presentations or demonstrations you know about.
| Date | Location | Event | Details |
|---|
Standards Organization - Professional Meetings/Conferences
| Date | Organization | Location | More Information |
|---|
See Also
Milestones for all IHE domains
Standard Organizations Link List
This page is based on the Domain Template.